当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Design-Enabled Divergent Modification of Monoterpene Synthases for Terpenoid Hyperproduction
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c05863 Liqiu Su, Pi Liu, Weidong Liu, Qi Liu, Jian Gao, Quanlu Zhao, Kaizhi Jia, Xiang Sheng, Hongwu Ma, Qinhong Wang, Zongjie Dai
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c05863 Liqiu Su, Pi Liu, Weidong Liu, Qi Liu, Jian Gao, Quanlu Zhao, Kaizhi Jia, Xiang Sheng, Hongwu Ma, Qinhong Wang, Zongjie Dai
|
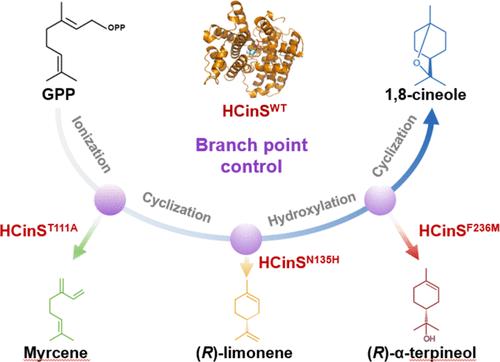
Enzymes’ catalytic promiscuity enables the alteration of product specificity via protein engineering; yet, harnessing this promiscuity to achieve desired catalytic reactions remains challenging. Here, we identified HCinS, a monoterpene synthase (MTPS) with a high efficiency and specificity for 1,8-cineole biosynthesis. Quantum mechanics/molecular mechanics (QM/MM) simulations, which were performed based on the resolved crystal structure of HCinS, revealed the mechanistic details of the biosynthetic cascade reactions. Guided by these insights, in silico HCinS variants were designed with fine-tuned transition-state energies and reaction microenvironments. Three variants (T111A, N135H, F236M), each with one amino acid substitution, exhibited high specificity in the production of monocyclic (R)-α-terpineol, (R)-limonene, and acyclic myrcene, respectively, maintaining over 55% efficiency of native HCinS. These designed HCinS variants surpassed naturally evolved isozymes in catalytic capacity and enabled yeast to achieve the highest microbial titer of each corresponding terpene. Furthermore, the single mutation of four functional equivalent amino acids in other four identified TPSs, respectively, resulted in the expected shifts on product specificity as HCinS variants. This research offers insights into the mechanisms controlling the TPS’s product promiscuity and highlights the universal applicability of computational design in reshaping the product specificity of TPSs, thereby paving innovative avenues for creating enzymes with applications in chemistry and synthetic biology.
中文翻译:

计算设计支持的单萜合酶发散修饰用于萜类化合物的高生成
酶的催化混杂能力能够通过蛋白质工程改变产品特异性;然而,利用这种混杂来实现所需的催化反应仍然具有挑战性。在这里,我们鉴定了 HCinS,一种对 1,8-桉树脑生物合成具有高效率和特异性的单萜合酶 (MTPS)。基于 HCinS 的分辨晶体结构进行的量子力学/分子力学 (QM/MM) 模拟揭示了生物合成级联反应的机理细节。在这些见解的指导下,计算机模拟 HCinS 变体被设计成具有微调的过渡态能量和反应微环境。三个变体 (T111A、N135H、F236M),每个变体都有一个氨基酸取代,分别在单环 (R)-α-松油醇、(R)-柠檬烯和无环月桂烯的产生中表现出高特异性,保持天然 HCinS 的效率超过 55%。这些设计的 HCinS 变体在催化能力上超过了自然进化的同工酶,并使酵母能够实现每个相应萜烯的最高微生物滴度。此外,其他四种已鉴定的 TPS 中四种功能等效氨基酸的单一突变分别导致 HCinS 变体的产品特异性发生预期变化。这项研究提供了对控制 TPS 产品混杂性的机制的见解,并强调了计算设计在重塑 TPS 产品特异性方面的普遍适用性,从而为创造在化学和合成生物学中应用的酶铺平了创新途径。
更新日期:2024-11-16
中文翻译:

计算设计支持的单萜合酶发散修饰用于萜类化合物的高生成
酶的催化混杂能力能够通过蛋白质工程改变产品特异性;然而,利用这种混杂来实现所需的催化反应仍然具有挑战性。在这里,我们鉴定了 HCinS,一种对 1,8-桉树脑生物合成具有高效率和特异性的单萜合酶 (MTPS)。基于 HCinS 的分辨晶体结构进行的量子力学/分子力学 (QM/MM) 模拟揭示了生物合成级联反应的机理细节。在这些见解的指导下,计算机模拟 HCinS 变体被设计成具有微调的过渡态能量和反应微环境。三个变体 (T111A、N135H、F236M),每个变体都有一个氨基酸取代,分别在单环 (R)-α-松油醇、(R)-柠檬烯和无环月桂烯的产生中表现出高特异性,保持天然 HCinS 的效率超过 55%。这些设计的 HCinS 变体在催化能力上超过了自然进化的同工酶,并使酵母能够实现每个相应萜烯的最高微生物滴度。此外,其他四种已鉴定的 TPS 中四种功能等效氨基酸的单一突变分别导致 HCinS 变体的产品特异性发生预期变化。这项研究提供了对控制 TPS 产品混杂性的机制的见解,并强调了计算设计在重塑 TPS 产品特异性方面的普遍适用性,从而为创造在化学和合成生物学中应用的酶铺平了创新途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号