当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Voltage-driven Molecular Switch of Highly Periodically N‒heterocyclic Adlayer Enabled Deep Cycled Zinc Metal Battery
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.ensm.2024.103910 Weina Xu, Bomian Zhang, Wangwang Xu, Guang Yao, Lei Zhang, Sitian Lian, Qi Liu, Chaozheng Liu, Ronghua Yuan, Wenzhou Chen, Xiaochang Qiao, Kangning Zhao
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.ensm.2024.103910 Weina Xu, Bomian Zhang, Wangwang Xu, Guang Yao, Lei Zhang, Sitian Lian, Qi Liu, Chaozheng Liu, Ronghua Yuan, Wenzhou Chen, Xiaochang Qiao, Kangning Zhao
|
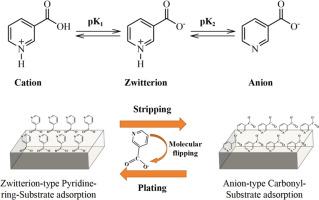
The instable Zn/electrolyte interface due to severe corrosion, especially at high utilization of Zn anode, strongly hindered the practical application of aqueous zinc metal battery. Herein, we report a voltage-driven molecular switch through a reversible transition of the nicotinic molecules between zwitterion and anion to enable deep cycled zinc metal battery. In light of in-situ Raman, the switching mechanism of nicotinic molecules in the electrical double layer is unveiled: during plating, nicotinic molecules switches to zwitterion mode (ON state) with periodical pyridine-ring-substrate adlayer while during stripping, shifts to anion mode with periodical carbonyl group-substrate adlayer (OFF state). The transition of NA molecules enables molecular flipping on the substrate due to the electrostatic force and in this way, in both ON and OFF state, the zinc anode is protected by the adlayer to avoid the zinc corrosion on the anode side. Furthermore, the OTF‒ decomposition is accompanied by the open ring reaction of N‒heterocyclic from nicotinic acid molecules to form highly elastic solid electrolyte interface layer. Benefiting from both the molecular switch function and solid electrolyte interface layer formation, the nicotinic molecules-based electrolyte enables practical zinc metal battery of high energy density (100 Wh/kgelectrode) for over 800 cycles with a cumulative capacity of 2.71 Ah cm−2 at practical condition of low N/P ratio of 2, and lean electrolyte of 10 μL mAh−1, representing the state-of-the-art performance. These findings highlight the utilization of molecular switch and its interfacial protection of the deep cycled zinc anode, and provide a new tactic for the development of high energy metal battery.
中文翻译:

高度周期性 N\u2012 杂环外加层启用的深度循环锌金属电池的电压驱动分子开关
由于严重腐蚀导致的 Zn/电解质界面不稳定,尤其是在 Zn 负极的高利用率下,强烈阻碍了水性锌金属电池的实际应用。在此,我们报道了一种电压驱动的分子开关,通过两性离子和阴离子之间的烟碱分子可逆转变来实现深度循环锌金属电池。根据原位拉曼,揭示了双电层中烟碱分子的开关机制:在电镀过程中,烟碱分子切换到具有周期性吡啶-环-衬底外加层的两性离子模式(ON 状态),而在剥离过程中,与周期性羰基-衬底外加层(OFF 状态)一起转变为阴离子模式。NA 分子的转变使分子在静电力的作用下在衬底上翻转,这样,在 ON 和 OFF 状态下,锌阳极都受到外加层的保护,以避免阳极侧的锌腐蚀。此外,OTF 分解伴随着烟酸分子 N\u2012 杂环的开环反应,形成高弹性的固体电解质界面层。得益于分子开关功能和固体电解质界面层的形成,基于烟碱分子的电解质使高能量密度的实用锌金属电池(100 Wh/kg电极)能够进行超过 800 次循环,累积容量为 2.71 Ah cm-2,在低 N/P 比为 2 和 10 μL mAh-1 的稀电解质的实际条件下, 代表最先进的性能。 这些发现突出了分子开关的利用及其对深循环锌负极的界面保护,为高能金属电池的开发提供了新的策略。
更新日期:2024-11-16
中文翻译:

高度周期性 N\u2012 杂环外加层启用的深度循环锌金属电池的电压驱动分子开关
由于严重腐蚀导致的 Zn/电解质界面不稳定,尤其是在 Zn 负极的高利用率下,强烈阻碍了水性锌金属电池的实际应用。在此,我们报道了一种电压驱动的分子开关,通过两性离子和阴离子之间的烟碱分子可逆转变来实现深度循环锌金属电池。根据原位拉曼,揭示了双电层中烟碱分子的开关机制:在电镀过程中,烟碱分子切换到具有周期性吡啶-环-衬底外加层的两性离子模式(ON 状态),而在剥离过程中,与周期性羰基-衬底外加层(OFF 状态)一起转变为阴离子模式。NA 分子的转变使分子在静电力的作用下在衬底上翻转,这样,在 ON 和 OFF 状态下,锌阳极都受到外加层的保护,以避免阳极侧的锌腐蚀。此外,OTF 分解伴随着烟酸分子 N\u2012 杂环的开环反应,形成高弹性的固体电解质界面层。得益于分子开关功能和固体电解质界面层的形成,基于烟碱分子的电解质使高能量密度的实用锌金属电池(100 Wh/kg电极)能够进行超过 800 次循环,累积容量为 2.71 Ah cm-2,在低 N/P 比为 2 和 10 μL mAh-1 的稀电解质的实际条件下, 代表最先进的性能。 这些发现突出了分子开关的利用及其对深循环锌负极的界面保护,为高能金属电池的开发提供了新的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号