当前位置:
X-MOL 学术
›
Energy Storage Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the nature of secondary phases in LiNi0.5Mn1.5O4 cathode materials using correlative Raman-SEM microscopy
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.ensm.2024.103905 Umair Nisar, Florian Klein, Claudia Pfeifer, Margret Wohlfahrt-Mehrens, Markus Hölzle, Peter Axmann
Energy Storage Materials ( IF 18.9 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.ensm.2024.103905 Umair Nisar, Florian Klein, Claudia Pfeifer, Margret Wohlfahrt-Mehrens, Markus Hölzle, Peter Axmann
|
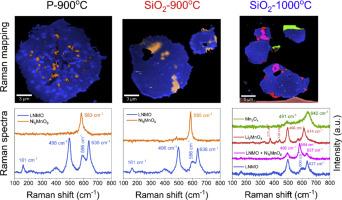
LiNi0.5 Mn1.5 O4 (LNMO) is a promising next-generation cathode material for lithium-ion batteries (LIBs) due to its high-energy and high-power density. However, its commercial adoption is hindered by the unstable LNMO/electrolyte interface due to high operating voltages and structural degradation arising from Jahn-Teller distortion and metal-ion dissolution resulting in poor cycling stability. Additionally, the high-temperature calcination beyond 700 °C often results in secondary phases such as rock salt NiO, Li1-x Nix O, Ni6 MnO8 or Li2 MnO3 , whose precise chemical compositions and their influence on electrochemical performance remain unclear. Traditional analytical techniques such as X-ray diffraction (XRD) or neutron diffraction face challenges in resolving these secondary phases due to low phase fractions and overlapping reflections with the LNMO phase. Here, we address these challenges using correlative Raman-Scanning electron microscopy (Raman-SEM) to characterize secondary phases in LNMO materials that were synthesized under various synthesis conditions and evaluated their impact on the electrochemical performance. Our results reveal the synthesis-dependent emergence of three distinct secondary phases in LNMO materials synthesized at 1000 °C, a phenomenon that, to our knowledge, has not been previously reported. Specifically, LNMO synthesized at 900 °C shows the coexistence of Ni6 MnO8 and Li2 MnO3 phases, while synthesized at 1000 °C also exhibits a Mn3 O4 phase. Furthermore, an increased amount of these secondary phases in LNMO led to a lower discharge capacity due to their electrochemical inactive nature. However, these phases do not negatively impact the rate capability or the long-term cycling performance of the LNMO materials. These insights are crucial for advancing the development of LNMO cathode materials for next-generation LIBs.
中文翻译:

使用相关拉曼-SEM 显微镜阐明 LiNi0.5Mn1.5O4 正极材料中第二相的性质
LiNi0.5Mn1.5O4 (LNMO) 因其高能量和高功率密度而成为一种很有前途的下一代锂离子电池 (LIB) 正极材料。然而,由于高工作电压以及 Jahn-Teller 畸变和金属离子溶解引起的结构退化,其商业采用受到不稳定的 LNMO/电解质界面的阻碍,导致循环稳定性差。此外,超过 700 °C 的高温煅烧通常会产生岩盐 NiO、Li1-xNixO、Ni6MnO8 或 Li2MnO3 等第二相,其精确的化学成分及其对电化学性能的影响尚不清楚。由于 X 射线衍射 (XRD) 或中子衍射等传统分析技术由于相分数低且与 LNMO 相的反射重叠,在解析这些二次相时面临挑战。在这里,我们使用相关拉曼扫描电子显微镜 (Raman-SEM) 来应对这些挑战,以表征在各种合成条件下合成的 LNMO 材料中的二次相,并评估它们对电化学性能的影响。我们的结果揭示了在 1000 °C 下合成的 LNMO 材料中出现三种不同的合成依赖性第二相,据我们所知,这种现象以前从未报道过。具体来说,在 900 °C 下合成的 LNMO 显示出 Ni6MnO8 和 Li2MnO3 相的共存,而在 1000 °C 下合成的 LNMO 也表现出 Mn3O4 相。此外,由于 LNMO 的电化学非活性性质,LNMO 中这些二次相的数量增加导致放电容量降低。然而,这些相不会对 LNMO 材料的倍率能力或长期循环性能产生负面影响。 这些见解对于推进用于下一代 LIB 的 LNMO 正极材料的开发至关重要。
更新日期:2024-11-16
中文翻译:

使用相关拉曼-SEM 显微镜阐明 LiNi0.5Mn1.5O4 正极材料中第二相的性质
LiNi0.5Mn1.5O4 (LNMO) 因其高能量和高功率密度而成为一种很有前途的下一代锂离子电池 (LIB) 正极材料。然而,由于高工作电压以及 Jahn-Teller 畸变和金属离子溶解引起的结构退化,其商业采用受到不稳定的 LNMO/电解质界面的阻碍,导致循环稳定性差。此外,超过 700 °C 的高温煅烧通常会产生岩盐 NiO、Li1-xNixO、Ni6MnO8 或 Li2MnO3 等第二相,其精确的化学成分及其对电化学性能的影响尚不清楚。由于 X 射线衍射 (XRD) 或中子衍射等传统分析技术由于相分数低且与 LNMO 相的反射重叠,在解析这些二次相时面临挑战。在这里,我们使用相关拉曼扫描电子显微镜 (Raman-SEM) 来应对这些挑战,以表征在各种合成条件下合成的 LNMO 材料中的二次相,并评估它们对电化学性能的影响。我们的结果揭示了在 1000 °C 下合成的 LNMO 材料中出现三种不同的合成依赖性第二相,据我们所知,这种现象以前从未报道过。具体来说,在 900 °C 下合成的 LNMO 显示出 Ni6MnO8 和 Li2MnO3 相的共存,而在 1000 °C 下合成的 LNMO 也表现出 Mn3O4 相。此外,由于 LNMO 的电化学非活性性质,LNMO 中这些二次相的数量增加导致放电容量降低。然而,这些相不会对 LNMO 材料的倍率能力或长期循环性能产生负面影响。 这些见解对于推进用于下一代 LIB 的 LNMO 正极材料的开发至关重要。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号