当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Length and Sequence-Selective Polymer Synthesis Templated by a Combination of Covalent and Noncovalent Base-Pairing Interactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-16 , DOI: 10.1021/jacs.4c13452 Federica Balduzzi, Vihanga Munasinghe, Oliver N. Evans, Agustin Lorusso Notaro Francesco, Cecilia J. Anderson, Salvatore Nigrelli, Luis Escobar, Rafel Cabot, Joseph T. Smith, Christopher A. Hunter
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-16 , DOI: 10.1021/jacs.4c13452 Federica Balduzzi, Vihanga Munasinghe, Oliver N. Evans, Agustin Lorusso Notaro Francesco, Cecilia J. Anderson, Salvatore Nigrelli, Luis Escobar, Rafel Cabot, Joseph T. Smith, Christopher A. Hunter
|
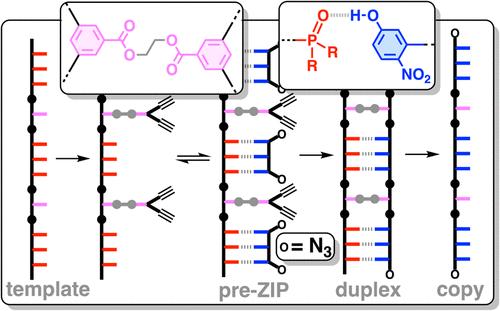
Information can be encoded and stored in sequences of monomer units organized in linear synthetic polymers. Replication of sequence information is of fundamental importance in biology; however, it represents a challenge for synthetic polymer chemistry. A combination of covalent and noncovalent base pairs has been used to achieve high-fidelity templated synthesis of synthetic polymers that encode information as a sequence of different side-chain recognition units. Dialkyne building blocks were attached to the template by using ester base pairs, and diazide building blocks were attached to the template by using H-bond base pairs. Copper-catalyzed azide–alkyne cycloaddition reactions were used to zip up the copy strand on the template, and the resulting duplex was cleaved by hydrolyzing the covalent ester base pairs. By using recognition-encoded melamine oligomers with either three phosphine oxide or three 4-nitrophenol recognition units to form the noncovalent base pairs, exceptionally high affinities of the diazides for the template were achieved, allowing the templated polymerization step to be carried out at low concentrations, which promoted on-template intramolecular reactions relative to competing intermolecular processes. Two different templates, a 7-mer and an 11-mer, were used in the three-step reaction sequence to obtain the sequence-complementary copy strands with minimal amounts of side reaction.
中文翻译:

通过共价和非共价碱基配对相互作用的组合模板化的长度和序列选择性聚合物合成
信息可以编码并存储在线性合成聚合物中组织的单体单元序列中。序列信息的复制在生物学中至关重要;然而,这对合成聚合物化学来说是一个挑战。共价和非共价碱基对的组合已被用于实现合成聚合物的高保真模板合成,该合成聚合物将信息编码为不同侧链识别单元的序列。二炔合成单元使用酯碱基对连接到模板上,二叠氮化物合成单元使用 H 键碱基对连接到模板上。使用铜催化的叠氮化物-炔烃环加成反应在模板上压缩复制链,并通过水解共价酯碱基对裂解所得双链体。通过使用具有三个氧化膦或三个 4-硝基苯酚识别单元的识别编码的三聚氰胺低聚物形成非共价碱基对,实现了模板二叠氮化物的极高亲和力,允许在低浓度下进行模板化聚合步骤,这促进了相对于竞争分子间过程的模板上分子内反应。在三步反应序列中使用了两种不同的模板,一种 7-mer 和一个 11-mer,以获得序列互补的拷贝链,而副反应量最少。
更新日期:2024-11-16
中文翻译:

通过共价和非共价碱基配对相互作用的组合模板化的长度和序列选择性聚合物合成
信息可以编码并存储在线性合成聚合物中组织的单体单元序列中。序列信息的复制在生物学中至关重要;然而,这对合成聚合物化学来说是一个挑战。共价和非共价碱基对的组合已被用于实现合成聚合物的高保真模板合成,该合成聚合物将信息编码为不同侧链识别单元的序列。二炔合成单元使用酯碱基对连接到模板上,二叠氮化物合成单元使用 H 键碱基对连接到模板上。使用铜催化的叠氮化物-炔烃环加成反应在模板上压缩复制链,并通过水解共价酯碱基对裂解所得双链体。通过使用具有三个氧化膦或三个 4-硝基苯酚识别单元的识别编码的三聚氰胺低聚物形成非共价碱基对,实现了模板二叠氮化物的极高亲和力,允许在低浓度下进行模板化聚合步骤,这促进了相对于竞争分子间过程的模板上分子内反应。在三步反应序列中使用了两种不同的模板,一种 7-mer 和一个 11-mer,以获得序列互补的拷贝链,而副反应量最少。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号