当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Carbyne Formation from C2H2 Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c07724 Miljan Z. Ćorović, Madeleine A. Ehweiner, Peter E. Hartmann, Felix Sbüll, Ferdinand Belaj, A. Daniel Boese, Jesse Lepluart, Martin L. Kirk, Nadia C. Mösch-Zanetti
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c07724 Miljan Z. Ćorović, Madeleine A. Ehweiner, Peter E. Hartmann, Felix Sbüll, Ferdinand Belaj, A. Daniel Boese, Jesse Lepluart, Martin L. Kirk, Nadia C. Mösch-Zanetti
|
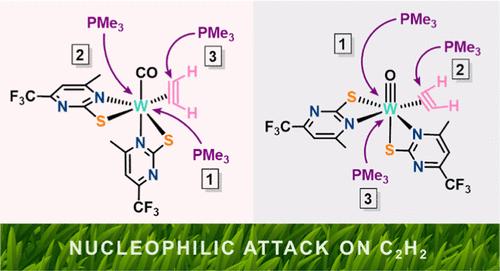
Nature chooses a high-valent tungsten center at the active site of the enzyme acetylene hydratase to facilitate acetylene hydration to acetaldehyde. However, the reactions of tungsten-coordinated acetylene are still not well understood, which prevents the development of sustainable bioinspired alkyne hydration catalysts. Here we report the reactivity of two bioinspired tungsten complexes with the acetylene ligand acting as a four-: [W(CO)(C2H2)(PymS)2] (1) and a two-electron donor: [WO(C2H2)(PymS)2] (3), with PMe3 as a nucleophile to simulate the enzyme’s reactivity (PymS = 4-(trifluoromethyl)-6-methylpyrimidine-2-thiolate). In dichloromethane, compound 1 was found to react to the cationic carbyne [W≡CCH2PMe3(CO)(PMe3)2(PymS)]Cl (2-Cl) while 3 reacts to the vinyl compound [WO(CH═CHPMe3)(PMe3)3(PymS)]Cl (4-Cl). The formation of the latter follows the common rules applied to η2-alkyne complexes, whereas the carbyne formation was not expected due to the challenging 1,2-H shift. To understand these differences in behavior between seemingly similar acetylene complexes, stepwise addition of the nucleophile in various solvents was investigated by synthetic, spectroscopic, and computational approaches. In this manuscript, we describe that only a four-electron donor acetylene complex can react to the carbyne over the η1-vinyl intermediate and that 1,2-H shift can be assisted by an H-transfer reagent (in this case, the decoordinated PymS ligand). Furthermore, to favor the attack of PMe3 at W coordinated acetylene, the metal center needs to be electron-poor and crowded enough to prevent nucleophile coordination. Finally, the intricate role of the anionic PymS ligand in the vicinity of the first coordination sphere models the potential involvement of amino acid residues during acetylene transformations in AH.
中文翻译:

了解 C2H2 配合物的 Carbyne Formation
大自然在乙炔水合酶的活性位点选择了一个高价钨中心,以促进乙炔水合为乙醛。然而,钨配位乙炔的反应仍未得到很好的理解,这阻碍了可持续生物启发炔烃水合催化剂的开发。在这里,我们报道了两种生物启发钨配合物的反应性,其中乙炔配体充当四-:[W(CO)(C2H2)(PymS)2] (1) 和一个双电子供体:[WO(C2H2)(PymS)2] (3),其中 PMe3 作为亲核试剂来模拟酶的反应性 (PymS = 4-(三氟甲基)-6-甲基嘧啶-2-硫酸盐)。在二氯甲烷中,发现化合物 1 与阳离子卡宾 [W≡CCH2PMe3(CO)(PMe3)2(PymS)]Cl (2-Cl) 反应,而化合物 3 与乙烯基化合物 [WO(CH═CHPMe3)(PMe3)3(PymS)]Cl (4-Cl) 反应。后者的形成遵循适用于η2-炔烃络合物的常见规则,而由于具有挑战性的 1,2-H 偏移,卡宾的形成是意料之中的。为了了解看似相似的乙炔配合物之间行为的这些差异,通过合成、光谱和计算方法研究了亲核试剂在各种溶剂中的逐步添加。在这份手稿中,我们描述了只有四电子供体乙炔配合物可以通过η1-乙烯基中间体与卡宾反应,并且 1,2-H 偏移可以由 H 转移试剂(在这种情况下,错位的 PymS 配体)辅助。 此外,为了有利于 PMe3 在 W 配位乙炔上的攻击,金属中心需要缺电子且足够拥挤,以防止亲核试剂配位。最后,阴离子 PymS 配体在第一配位球附近的复杂作用模拟了 AH 中乙炔转化过程中氨基酸残基的潜在参与。
更新日期:2024-11-16
中文翻译:

了解 C2H2 配合物的 Carbyne Formation
大自然在乙炔水合酶的活性位点选择了一个高价钨中心,以促进乙炔水合为乙醛。然而,钨配位乙炔的反应仍未得到很好的理解,这阻碍了可持续生物启发炔烃水合催化剂的开发。在这里,我们报道了两种生物启发钨配合物的反应性,其中乙炔配体充当四-:[W(CO)(C2H2)(PymS)2] (1) 和一个双电子供体:[WO(C2H2)(PymS)2] (3),其中 PMe3 作为亲核试剂来模拟酶的反应性 (PymS = 4-(三氟甲基)-6-甲基嘧啶-2-硫酸盐)。在二氯甲烷中,发现化合物 1 与阳离子卡宾 [W≡CCH2PMe3(CO)(PMe3)2(PymS)]Cl (2-Cl) 反应,而化合物 3 与乙烯基化合物 [WO(CH═CHPMe3)(PMe3)3(PymS)]Cl (4-Cl) 反应。后者的形成遵循适用于η2-炔烃络合物的常见规则,而由于具有挑战性的 1,2-H 偏移,卡宾的形成是意料之中的。为了了解看似相似的乙炔配合物之间行为的这些差异,通过合成、光谱和计算方法研究了亲核试剂在各种溶剂中的逐步添加。在这份手稿中,我们描述了只有四电子供体乙炔配合物可以通过η1-乙烯基中间体与卡宾反应,并且 1,2-H 偏移可以由 H 转移试剂(在这种情况下,错位的 PymS 配体)辅助。 此外,为了有利于 PMe3 在 W 配位乙炔上的攻击,金属中心需要缺电子且足够拥挤,以防止亲核试剂配位。最后,阴离子 PymS 配体在第一配位球附近的复杂作用模拟了 AH 中乙炔转化过程中氨基酸残基的潜在参与。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号