当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Insights into the Mechanism of a Polyketide Synthase Thiocysteine Lyase Domain
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c11656 Andrew D. Steele, Song Meng, Gengnan Li, Edward Kalkreuter, Changsoo Chang, Ben Shen
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c11656 Andrew D. Steele, Song Meng, Gengnan Li, Edward Kalkreuter, Changsoo Chang, Ben Shen
|
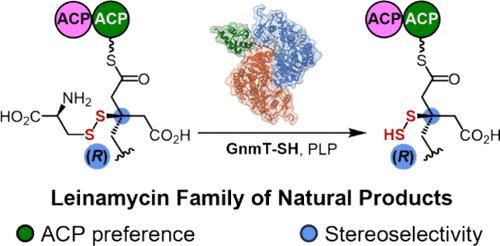
Polyketide synthases (PKSs) are renowned for the structural diversity of the polyketide natural products they produce, but sulfur-containing functionalities are rarely installed by PKSs. We previously characterized thiocysteine lyase (SH) domains involved in the biosynthesis of the leinamycin (LNM) family of natural products, exemplified by LnmJ-SH and guangnanmycin (GnmT-SH). Here we report a detailed investigation into the PLP-dependent reaction catalyzed by the SH domains, guided by a 1.8 Å resolution crystal structure of GnmT-SH. A series of elaborate substrate mimics were synthesized to answer specific questions garnered from the crystal structure and from the biosynthetic logic of the LNM family of natural products. Through a combination of bioinformatics, molecular modeling, in vitro assays, and mutagenesis, we have developed a detailed model of acyl carrier protein (ACP)-tethered substrate-SH, and interdomain interactions, that contribute to the observed substrate specificity. Comparison of the GnmT-SH structure with archetypical PLP-dependent enzyme structures revealed how Nature, via evolution, has modified a common protein structural motif to accommodate an ACP-tethered substrate, which is significantly larger than any of those previously characterized. Overall, this study demonstrates how PLP-dependent chemistry can be incorporated into the context of PKS assembly lines and sets the stage for engineering PKSs to produce sulfur-containing polyketides.
中文翻译:

聚酮合酶硫代半胱氨酸裂解酶结构域机制的结构见解
聚酮合酶 (PKS) 以其产生的聚酮天然产物的结构多样性而闻名,但 PKS 很少安装含硫官能团。我们之前表征了参与天然产物 leinamycin (LNM) 家族生物合成的硫代半胱氨酸裂解酶 (SH) 结构域,以 LnmJ-SH 和广南霉素 (GnmT-SH) 为例。在这里,我们报告了在 GnmT-SH 的 1.8 Å 分辨率晶体结构的指导下,对 SH 结构域催化的 PLP 依赖性反应的详细研究。合成了一系列精心设计的底物模拟物,以回答从 LNM 天然产物家族的晶体结构和生物合成逻辑中获得的具体问题。通过结合生物信息学、分子建模、体外测定和诱变,我们开发了酰基载体蛋白 (ACP) 栓系底物-SH 和结构域间相互作用的详细模型,这有助于观察到底物特异性。将 GnmT-SH 结构与原型 PLP 依赖性酶结构进行比较,揭示了大自然如何通过进化修饰常见的蛋白质结构基序以适应 ACP 栓系底物,该底物明显大于先前表征的任何底物。总体而言,本研究展示了如何将 PLP 依赖性化学成分整合到 PKS 装配线的环境中,并为工程 PKS 生产含硫聚酮奠定了基础。
更新日期:2024-11-16
中文翻译:

聚酮合酶硫代半胱氨酸裂解酶结构域机制的结构见解
聚酮合酶 (PKS) 以其产生的聚酮天然产物的结构多样性而闻名,但 PKS 很少安装含硫官能团。我们之前表征了参与天然产物 leinamycin (LNM) 家族生物合成的硫代半胱氨酸裂解酶 (SH) 结构域,以 LnmJ-SH 和广南霉素 (GnmT-SH) 为例。在这里,我们报告了在 GnmT-SH 的 1.8 Å 分辨率晶体结构的指导下,对 SH 结构域催化的 PLP 依赖性反应的详细研究。合成了一系列精心设计的底物模拟物,以回答从 LNM 天然产物家族的晶体结构和生物合成逻辑中获得的具体问题。通过结合生物信息学、分子建模、体外测定和诱变,我们开发了酰基载体蛋白 (ACP) 栓系底物-SH 和结构域间相互作用的详细模型,这有助于观察到底物特异性。将 GnmT-SH 结构与原型 PLP 依赖性酶结构进行比较,揭示了大自然如何通过进化修饰常见的蛋白质结构基序以适应 ACP 栓系底物,该底物明显大于先前表征的任何底物。总体而言,本研究展示了如何将 PLP 依赖性化学成分整合到 PKS 装配线的环境中,并为工程 PKS 生产含硫聚酮奠定了基础。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号