当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterogenous Chemistry of I2O3 as a Critical Step in Iodine Cycling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c13060 An Ning, Jing Li, Lin Du, Xiaohua Yang, Jiarong Liu, Zhi Yang, Jie Zhong, Alfonso Saiz-Lopez, Ling Liu, Joseph S. Francisco, Xiuhui Zhang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c13060 An Ning, Jing Li, Lin Du, Xiaohua Yang, Jiarong Liu, Zhi Yang, Jie Zhong, Alfonso Saiz-Lopez, Ling Liu, Joseph S. Francisco, Xiuhui Zhang
|
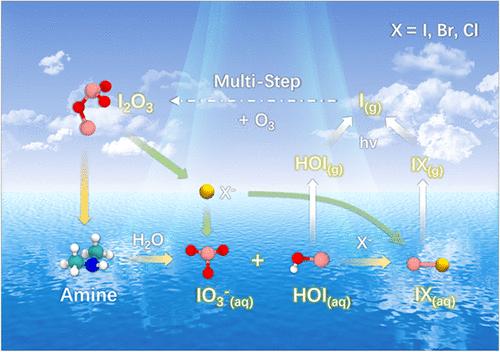
Global iodine emissions have been increasing rapidly in recent decades, further influencing the Earth’s climate and human health. However, our incomplete understanding of the iodine chemical cycle, especially the fate of higher iodine oxides, introduces substantial uncertainties into atmospheric modeling. I2O3 was previously deemed a “dead end” in iodine chemistry; however, we provide atomic-level evidence that I2O3 can undergo rapid air–water or air–ice interfacial reactions within several picoseconds; these reactions are facilitated by prevalent chemicals on seawater such as amines and halide ions, to produce photolabile reactive iodine species such as HOI and IX (X = I, Br, and Cl). The heterogeneous chemistry of I2O3 leads to the rapid formation of iodate ions (IO3–), which is the predominant soluble iodine and its concentration cannot be well explained by current chemistry. These new loss pathways for atmospheric I2O3 can further explain its absence in field observations and its presence in laboratory experiments; furthermore, these pathways represent a heterogeneous recycling mechanism that can activate the release of reactive iodine from oceans, polar ice/snowpack, or aerosols. Rapid reactive adsorption of I2O3 can also promote the growth of marine aerosols. These findings provide novel insights into iodine geochemical cycling.
中文翻译:

I2O3 的非均相化学是碘循环的关键步骤
近几十年来,全球碘排放量迅速增加,进一步影响了地球的气候和人类健康。然而,我们对碘化学循环的理解不完整,尤其是对高碘氧化物的命运,给大气建模带来了很大的不确定性。I2O3 以前被认为是碘化学中的“死胡同”;然而,我们提供了原子级证据表明 I2O3 可以在几皮秒内发生快速的空气-水或空气-冰界面反应;海水中普遍存在的化学物质(如胺和卤化物离子)促进了这些反应,以产生不易光敏的反应性碘物质,如 HOI 和 IX(X = I、Br 和 Cl)。I2O3 的异质化学导致碘离子 (IO3–) 的快速形成,碘离子是主要的可溶性碘,其浓度不能用当前的化学成分很好地解释。大气 I2O3 的这些新损失途径可以进一步解释它在野外观测中的缺失和在实验室实验中的存在;此外,这些途径代表了一种异质循环机制,可以激活海洋、极地冰/积雪或气溶胶中活性碘的释放。I2O3 的快速反应吸附也可促进海洋气溶胶的生长。这些发现为碘地球化学循环提供了新的见解。
更新日期:2024-11-16
中文翻译:

I2O3 的非均相化学是碘循环的关键步骤
近几十年来,全球碘排放量迅速增加,进一步影响了地球的气候和人类健康。然而,我们对碘化学循环的理解不完整,尤其是对高碘氧化物的命运,给大气建模带来了很大的不确定性。I2O3 以前被认为是碘化学中的“死胡同”;然而,我们提供了原子级证据表明 I2O3 可以在几皮秒内发生快速的空气-水或空气-冰界面反应;海水中普遍存在的化学物质(如胺和卤化物离子)促进了这些反应,以产生不易光敏的反应性碘物质,如 HOI 和 IX(X = I、Br 和 Cl)。I2O3 的异质化学导致碘离子 (IO3–) 的快速形成,碘离子是主要的可溶性碘,其浓度不能用当前的化学成分很好地解释。大气 I2O3 的这些新损失途径可以进一步解释它在野外观测中的缺失和在实验室实验中的存在;此外,这些途径代表了一种异质循环机制,可以激活海洋、极地冰/积雪或气溶胶中活性碘的释放。I2O3 的快速反应吸附也可促进海洋气溶胶的生长。这些发现为碘地球化学循环提供了新的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号