当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Mechanistic Study of Naphthalimide Derivative Formation via Cu-Mediated Ullmann-Type Reactions
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.inorgchem.4c03688 Azita Noshirvani Sharifabad, Alireza Khosravi, Farzad Kobarfard
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.inorgchem.4c03688 Azita Noshirvani Sharifabad, Alireza Khosravi, Farzad Kobarfard
|
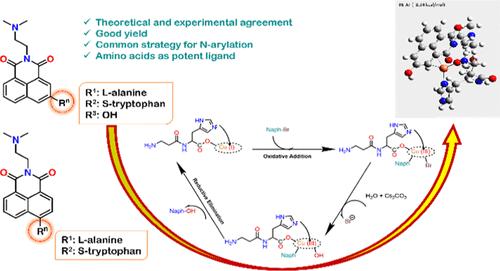
Cu-mediated Ullmann-type coupling reactions are fundamental to organic synthesis, garnering significant academic and industrial interest since their inception. Optimizing reaction parameters, particularly temperature control, is crucial for maximizing efficiency while maintaining high yields. Bidentate ligands, such as amino acids, have demonstrated potential in facilitating these reactions at lower temperatures (<100 °C). This study explores the Cu-catalyzed Ullmann-type coupling of naphthalimide derivatives with amino acid substitutions. Naphthalimide dyes, known for their diverse applications in bioimaging, solar cells, medicine, and sensors, were selected for their potent anticancer properties. The synthesized compounds were characterized by using 1H NMR, ESI-MS, and melting point analyses. Compounds with significant steric hindrance exhibited lower yields, leading to the development of a novel catalytic system employing l-carnosine as a bidentate ligand, which significantly improved yields. Mechanistic insights, derived from density functional theory calculations, identified “L-complex 3” as the most stable and reactive intermediate during oxidative addition to aryl halides. The oxidative addition transition state “OX1-TS” was found to be the most favorable, with a relatively low energy barrier of 6.13 kcal/mol, suggesting that this step, despite being the rate-limiting stage, is energetically accessible. In contrast, reductive elimination was facilitated by a Cu(III) penta-coordinated intermediate, with a barrier of just 8.34 kcal/mol, making it a more straightforward process. Theoretical findings aligned closely with experimental data, reinforcing the oxidative addition/reductive elimination pathway as the operative mechanism for this reaction.
中文翻译:

Cu介导的Ullmann型反应形成萘苯二酰亚胺衍生物的合成及机理研究
Cu 介导的 Ullmann 型偶联反应是有机合成的基础,自诞生以来就引起了学术界和工业界的极大兴趣。优化反应参数,尤其是温度控制,对于在保持高产量的同时最大限度地提高效率至关重要。二齿配体,如氨基酸,已显示出在较低温度 (<100 °C) 下促进这些反应的潜力。本研究探讨了 Cu 催化的萘酰亚胺衍生物与氨基酸取代的 Ullmann 型偶联。萘酞酰亚胺染料以其在生物成像、太阳能电池、医学和传感器中的多种应用而闻名,因其强大的抗癌特性而被选中。使用 1H NMR、ESI-MS 和熔点分析对合成的化合物进行表征。具有显著空间位阻的化合物表现出较低的产率,导致开发了一种采用 l-肌肽作为双齿配体的新型催化系统,从而显著提高了产率。从密度泛函理论计算得出的机理见解确定“L-复合物 3”是芳基卤化物氧化加成过程中最稳定和反应性最强的中间体。发现氧化加成过渡态 “OX1-TS” 是最有利的,具有 6.13 kcal/mol 的相对较低的能垒,这表明这一步尽管是限速阶段,但在能量上是可及的。相比之下,Cu(III) 五配位中间体促进了还原消除,屏障仅为 8.34 kcal/mol,使其成为一个更直接的过程。 理论发现与实验数据密切相关,加强了氧化加成/还原消除途径作为该反应的运作机制。
更新日期:2024-11-15
中文翻译:

Cu介导的Ullmann型反应形成萘苯二酰亚胺衍生物的合成及机理研究
Cu 介导的 Ullmann 型偶联反应是有机合成的基础,自诞生以来就引起了学术界和工业界的极大兴趣。优化反应参数,尤其是温度控制,对于在保持高产量的同时最大限度地提高效率至关重要。二齿配体,如氨基酸,已显示出在较低温度 (<100 °C) 下促进这些反应的潜力。本研究探讨了 Cu 催化的萘酰亚胺衍生物与氨基酸取代的 Ullmann 型偶联。萘酞酰亚胺染料以其在生物成像、太阳能电池、医学和传感器中的多种应用而闻名,因其强大的抗癌特性而被选中。使用 1H NMR、ESI-MS 和熔点分析对合成的化合物进行表征。具有显著空间位阻的化合物表现出较低的产率,导致开发了一种采用 l-肌肽作为双齿配体的新型催化系统,从而显著提高了产率。从密度泛函理论计算得出的机理见解确定“L-复合物 3”是芳基卤化物氧化加成过程中最稳定和反应性最强的中间体。发现氧化加成过渡态 “OX1-TS” 是最有利的,具有 6.13 kcal/mol 的相对较低的能垒,这表明这一步尽管是限速阶段,但在能量上是可及的。相比之下,Cu(III) 五配位中间体促进了还原消除,屏障仅为 8.34 kcal/mol,使其成为一个更直接的过程。 理论发现与实验数据密切相关,加强了氧化加成/还原消除途径作为该反应的运作机制。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号