当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Hydrophobic Property of Blue Fluorescent Amino Acid 4-Cyanotryptophan
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpclett.4c02842 Manxi Wang, Bo Zhuang, Kailin Tang, Ran-ran Feng, Feng Gai
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpclett.4c02842 Manxi Wang, Bo Zhuang, Kailin Tang, Ran-ran Feng, Feng Gai
|
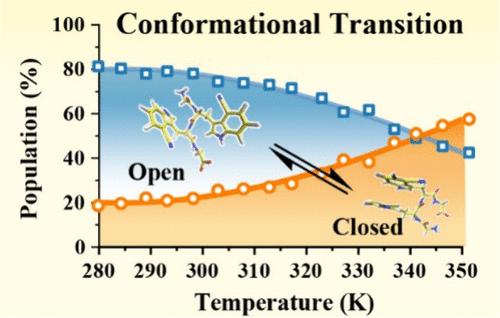
It is a common belief that the negative heat capacity change (ΔCp) associated with protein folding, which is a manifestation of the hydrophobic effect, results from a decrease in the solvent accessible hydrophobic surface area. Herein, we investigate the conformational energy landscape and dynamics of a tetrapeptide composed of two glycine and two 4-cyanotryptophan residues using time-resolved fluorescence spectroscopy, molecular dynamics simulations, and density functional theory calculations and find that, contrary to this expectation, the hydrophobic association of two 4-cyanotryptophan side chains leads to a positive ΔCp (approximately 543 J K–1 mol–1). Furthermore, we find that promoting one of the 4-cyanotryptophans to its excited electronic state strengthens this self-association. Taken together, our results provide not only insight into how modification of an aromatic amino acid can affect its hydrophobicity but also a potential strategy for designing protein sequences that can fold (unfold) at high (low) temperatures.
中文翻译:

蓝色荧光氨基酸 4-氰色氨酸的不寻常疏水特性
人们普遍认为,与蛋白质折叠相关的负热容变化 (ΔCp) 是疏水效应的一种表现,是由于溶剂可接近的疏水表面积减少造成的。在本文中,我们使用时间分辨荧光光谱、分子动力学模拟和密度泛函理论计算研究了由两个甘氨酸和两个 4-氰色氨酸残基组成的四肽的构象能量图和动力学,发现与这一预期相反,两个 4-氰色氨酸侧链的疏水缔合导致正 ΔCp(约 543 J K–1 mol–1).此外,我们发现将 4-氰色氨酸之一提升到其激发的电子状态会增强这种自缔合。综上所述,我们的结果不仅提供了关于芳香族氨基酸修饰如何影响其疏水性的见解,而且还提供了设计可以在高(低)温度下折叠(展开)的蛋白质序列的潜在策略。
更新日期:2024-11-16
中文翻译:

蓝色荧光氨基酸 4-氰色氨酸的不寻常疏水特性
人们普遍认为,与蛋白质折叠相关的负热容变化 (ΔCp) 是疏水效应的一种表现,是由于溶剂可接近的疏水表面积减少造成的。在本文中,我们使用时间分辨荧光光谱、分子动力学模拟和密度泛函理论计算研究了由两个甘氨酸和两个 4-氰色氨酸残基组成的四肽的构象能量图和动力学,发现与这一预期相反,两个 4-氰色氨酸侧链的疏水缔合导致正 ΔCp(约 543 J K–1 mol–1).此外,我们发现将 4-氰色氨酸之一提升到其激发的电子状态会增强这种自缔合。综上所述,我们的结果不仅提供了关于芳香族氨基酸修饰如何影响其疏水性的见解,而且还提供了设计可以在高(低)温度下折叠(展开)的蛋白质序列的潜在策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号