当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu-Supported ZnO under Conditions of CO2 Reduction to Methanol: Why 0.2 ML Coverage?
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpclett.4c02908 Robert H. Lavroff, Edison Cummings, Kaustubh Sawant, Zisheng Zhang, Philippe Sautet, Anastassia N. Alexandrova
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpclett.4c02908 Robert H. Lavroff, Edison Cummings, Kaustubh Sawant, Zisheng Zhang, Philippe Sautet, Anastassia N. Alexandrova
|
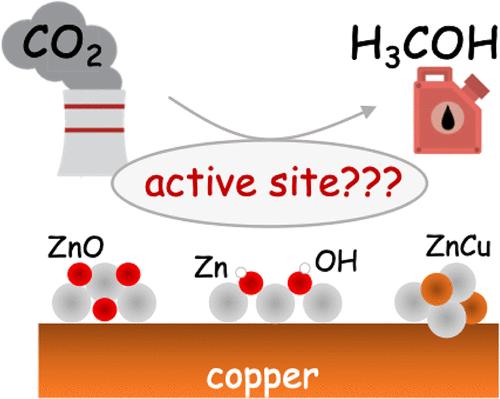
By hydrogenating carbon dioxide to value-added products such as methanol, heterogeneous catalysts can lower greenhouse gas emissions and generate alternative liquid fuels. The most common commercial catalyst for the reduction of CO2 to methanol is Cu/ZnO/Al2O3, where ZnO improves conversion and selectivity toward methanol. The structure of this catalyst is thought to be Zn oxy(hydroxyl) overlayers on the nanometer scale on Cu. In the presence of CO2 and H2 under reaction conditions, the Cu substrate itself can be restructured and/or partially oxidized at its interface with ZnO, or the Zn might be reduced, possibly completely to a CuZn alloy, making the exact structure and stoichiometry of the active site a topic of active debate. In this study, we examine Zn3 clusters on Cu(100) and Cu(111), as a subnano model of the catalyst. We use a grand canonical genetic algorithm to sample the system structure and stoichiometry under catalytic conditions: T of 550 K, initial partial pressures of H2 of 4.5 atm and CO2 of 0.5 atm, and 1% conversion. We uncover a strong dependence of the catalyst stoichiometry on the surface coverage. At the optimal 0.2 ML surface coverage, chains of Zn(OH) form on both Cu surfaces. On Cu(100), the catalyst has many thermally accessible metastable minima, whereas on Cu(111), it does not. No oxidation or reconstruction of the Cu is found. However, at a lower coverage of Zn, Zn3 clusters take on a metallic form on Cu(100), and slightly oxidized Zn3O on Cu(111), while the surface uptakes H to form a variety of low hydrides of Cu. We thus hypothesize that the 0.2 ML Zn coverage is optimal, as found experimentally, because of the stronger yet incomplete oxidation afforded by Zn at this coverage.
中文翻译:

在 CO2 还原为甲醇的条件下,铜负载的 ZnO:为什么覆盖率为 0.2 ML?
通过将二氧化碳加氢成甲醇等增值产品,非均相催化剂可以降低温室气体排放并产生替代液体燃料。将 CO2 还原为甲醇的最常见商业催化剂是 Cu/ZnO/Al2O3,其中 ZnO 提高了对甲醇的转化率和选择性。这种催化剂的结构被认为是 Cu 上纳米尺度上的 Zn 氧(羟基)覆盖层。在反应条件下存在 CO2 和 H2 的情况下,Cu 衬底本身可以在其与 ZnO 的界面处重组和/或部分氧化,或者 Zn 可能被还原,可能完全转化为 CuZn 合金,这使得活性位点的确切结构和化学计量成为一个激烈争论的话题。在这项研究中,我们研究了 Cu(100) 和 Cu(111) 上的 Zn3 团簇作为催化剂的亚纳米模型。我们使用大规范遗传算法对催化条件下的系统结构和化学计量进行采样:T 为 550 K,H2 的初始分压为 4.5 atm,CO2 为 0.5 atm,转化率为 1%。我们揭示了催化剂化学计量对表面覆盖率的强烈依赖性。在最佳 0.2 ML 表面覆盖率下,Zn(OH) 链在两个 Cu 表面上形成。在 Cu(100) 上,催化剂具有许多热可及的亚稳最小值,而在 Cu(111) 上则没有。未发现 Cu 的氧化或重建。然而,在 Zn 覆盖率较低的情况下,Zn3 团簇在 Cu(100) 上呈金属形式,在 Cu(111) 上呈轻微氧化的 Zn3O,而表面吸收 H 形成各种低氢化物的 Cu。因此,我们假设 0.正如实验发现的那样,2 ML Zn 覆盖率是最佳的,因为在此覆盖率下 Zn 提供了更强但不完全的氧化。
更新日期:2024-11-16
中文翻译:

在 CO2 还原为甲醇的条件下,铜负载的 ZnO:为什么覆盖率为 0.2 ML?
通过将二氧化碳加氢成甲醇等增值产品,非均相催化剂可以降低温室气体排放并产生替代液体燃料。将 CO2 还原为甲醇的最常见商业催化剂是 Cu/ZnO/Al2O3,其中 ZnO 提高了对甲醇的转化率和选择性。这种催化剂的结构被认为是 Cu 上纳米尺度上的 Zn 氧(羟基)覆盖层。在反应条件下存在 CO2 和 H2 的情况下,Cu 衬底本身可以在其与 ZnO 的界面处重组和/或部分氧化,或者 Zn 可能被还原,可能完全转化为 CuZn 合金,这使得活性位点的确切结构和化学计量成为一个激烈争论的话题。在这项研究中,我们研究了 Cu(100) 和 Cu(111) 上的 Zn3 团簇作为催化剂的亚纳米模型。我们使用大规范遗传算法对催化条件下的系统结构和化学计量进行采样:T 为 550 K,H2 的初始分压为 4.5 atm,CO2 为 0.5 atm,转化率为 1%。我们揭示了催化剂化学计量对表面覆盖率的强烈依赖性。在最佳 0.2 ML 表面覆盖率下,Zn(OH) 链在两个 Cu 表面上形成。在 Cu(100) 上,催化剂具有许多热可及的亚稳最小值,而在 Cu(111) 上则没有。未发现 Cu 的氧化或重建。然而,在 Zn 覆盖率较低的情况下,Zn3 团簇在 Cu(100) 上呈金属形式,在 Cu(111) 上呈轻微氧化的 Zn3O,而表面吸收 H 形成各种低氢化物的 Cu。因此,我们假设 0.正如实验发现的那样,2 ML Zn 覆盖率是最佳的,因为在此覆盖率下 Zn 提供了更强但不完全的氧化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号