当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydroxyl-Induced Modification of Oxygen Activation and Desorption Free Energy on Defective Tetragonal Zirconia Catalysts
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpcc.4c04866 Sara Fazeli, Pascal Brault, Amaël Caillard, Eric Millon
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.jpcc.4c04866 Sara Fazeli, Pascal Brault, Amaël Caillard, Eric Millon
|
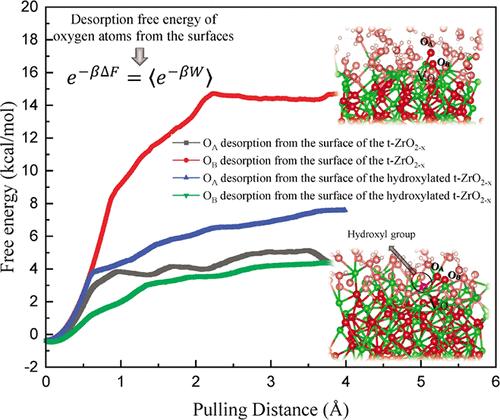
Describing the activation of O2 on metal surfaces is crucial for understanding fundamental electrochemical processes, such as the oxygen reduction reaction (ORR) in hydrogen fuel cells. This study explores how defects influence O2 adsorption mechanisms on a zirconia-based cathode. In the first step, we model O2 adsorption on two defective surfaces: oxygen-deficient t-ZrO2–x and oxynitride t-ZrO2–xNx, in an aqueous solution. We describe various O2 adsorption states by analyzing charge transfer and cohesive energy changes in O2 molecules, Zr active sites, and defects. The results suggest that O2 adsorption mechanisms on the surfaces of t-ZrO2–x and t-ZrO2–xNx occur through dissociative and associative pathways, respectively. Additionally, O2 adsorption on t-ZrO2–xNx leads to the departure of N dopants from the surface, which is unfavorable for catalytic activity. In the second step, we modified the surfaces of t-ZrO2–x and t-ZrO2–xNx with the hydroxyl (OH) group. Afterward, we simulate the O2 activation process on these modified surfaces and identify the most probable active sites. Our findings reveal that OH groups stabilize N dopants on hydroxylated t-ZrO2–xNx, preventing their loss. Moreover, OH groups influence the O2 adsorption mechanism on t-ZrO2–x, shifting toward associative O–O bond breaking. Conversely, O2 adsorption on hydroxylated t-ZrO2–xNx remains molecularly associative. Overall, on hydroxylated surfaces, O2 adsorption involves stronger charge transfer among oxygen, defects, and Zr active sites. In the third step, we explored the trends of desorption of the O2 from these surfaces. This entails analyzing O2 desorption using steered molecular dynamics (SMD) to generate potential mean force (PMF) profiles and applying Jarzynski’s equality to calculate the free energy of desorption. Herein, we find that the free energy of the desorption of O2 from hydroxylated surfaces is lower, indicating a more spontaneous process compared to t-ZrO2–x and t-ZrO2–xNx. Moreover, we discover that oxygen has the highest tendency to desorb from the hydroxylated-ZrO2–x surface, which is attributed to the lowest free energy involved in pulling oxygen from the surface, potentially influencing ORR acceleration. These findings offer valuable guidance for developing efficient nonplatinum-based cathode materials, particularly in catalysis applications.
中文翻译:

羟基诱导的氧化氢活化和解吸自由能对缺陷四方氧化锆催化剂的改性
描述金属表面 O2 的活化对于理解基本的电化学过程至关重要,例如氢燃料电池中的氧还原反应 (ORR)。本研究探讨了缺陷如何影响氧化锆基阴极上的 O2 吸附机制。第一步,我们在水溶液中模拟了 O2 在两个有缺陷的表面上的吸附:缺氧 t-ZrO 2-x 和氮氧化物 t-ZrO 2-xNx。我们通过分析 O2 分子、Zr 活性位点和缺陷中的电荷转移和内聚能变化来描述各种 O2 吸附态。结果表明,t-ZrO 2-x 和 t-ZrO 2-xNx 表面的 O2 吸附机制分别通过解离和缔合途径发生。此外,O2 吸附在 t-ZrO2–xNx 上导致 N 掺杂剂离开表面,这对催化活性不利。在第二步中,我们用羟基 (OH) 基团修饰了 t-ZrO 2-x 和 t-ZrO 2-xNx 的表面。之后,我们在这些修饰的表面上模拟 O2 活化过程并确定最可能的活性位点。我们的研究结果表明,OH 基团稳定羟基化 t-ZrO 2-xNx 上的 N 掺杂剂,防止其丢失。此外,OH 基团影响 t-ZrO 2-x 上的 O2 吸附机制,转向缔合 O-O 键断裂。 相反,O2 在羟基化的 t-ZrO 2-xNx 上的吸附保持分子缔合。总体而言,在羟基化表面上,O2 吸附涉及氧、缺陷和 Zr 活性位点之间更强的电荷转移。第三步,我们探索了 O2 从这些表面解吸的趋势。这需要使用受控分子动力学 (SMD) 分析 O2 解吸以生成势平均力 (PMF) 曲线,并应用 Jarzynski 等式来计算解吸的自由能。在此,我们发现 O2 从羟基化表面解吸的自由能较低,表明与 t-ZrO 2-x 和 t-ZrO 2-xNx 相比,过程更加自发。此外,我们发现氧具有最高的从羟基化 ZrO 2-x 表面解吸的倾向,这归因于从表面拉出氧所涉及的最低自由能,这可能会影响 ORR 加速。这些发现为开发高效的非铂基正极材料提供了有价值的指导,尤其是在催化应用中。
更新日期:2024-11-16
中文翻译:

羟基诱导的氧化氢活化和解吸自由能对缺陷四方氧化锆催化剂的改性
描述金属表面 O2 的活化对于理解基本的电化学过程至关重要,例如氢燃料电池中的氧还原反应 (ORR)。本研究探讨了缺陷如何影响氧化锆基阴极上的 O2 吸附机制。第一步,我们在水溶液中模拟了 O2 在两个有缺陷的表面上的吸附:缺氧 t-ZrO 2-x 和氮氧化物 t-ZrO 2-xNx。我们通过分析 O2 分子、Zr 活性位点和缺陷中的电荷转移和内聚能变化来描述各种 O2 吸附态。结果表明,t-ZrO 2-x 和 t-ZrO 2-xNx 表面的 O2 吸附机制分别通过解离和缔合途径发生。此外,O2 吸附在 t-ZrO2–xNx 上导致 N 掺杂剂离开表面,这对催化活性不利。在第二步中,我们用羟基 (OH) 基团修饰了 t-ZrO 2-x 和 t-ZrO 2-xNx 的表面。之后,我们在这些修饰的表面上模拟 O2 活化过程并确定最可能的活性位点。我们的研究结果表明,OH 基团稳定羟基化 t-ZrO 2-xNx 上的 N 掺杂剂,防止其丢失。此外,OH 基团影响 t-ZrO 2-x 上的 O2 吸附机制,转向缔合 O-O 键断裂。 相反,O2 在羟基化的 t-ZrO 2-xNx 上的吸附保持分子缔合。总体而言,在羟基化表面上,O2 吸附涉及氧、缺陷和 Zr 活性位点之间更强的电荷转移。第三步,我们探索了 O2 从这些表面解吸的趋势。这需要使用受控分子动力学 (SMD) 分析 O2 解吸以生成势平均力 (PMF) 曲线,并应用 Jarzynski 等式来计算解吸的自由能。在此,我们发现 O2 从羟基化表面解吸的自由能较低,表明与 t-ZrO 2-x 和 t-ZrO 2-xNx 相比,过程更加自发。此外,我们发现氧具有最高的从羟基化 ZrO 2-x 表面解吸的倾向,这归因于从表面拉出氧所涉及的最低自由能,这可能会影响 ORR 加速。这些发现为开发高效的非铂基正极材料提供了有价值的指导,尤其是在催化应用中。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号