当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective Dehydrogenation and Hetero-Arylation of Amides via Radical Translocation Enabled by Photoexcited Triplet Ketone Catalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-16 , DOI: 10.1021/acs.joc.4c02060 Akash Bisoyi, Amit Behera, Alisha Rani Tripathy, Vijay Kumar Simhadri, Veera Reddy Yatham
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-16 , DOI: 10.1021/acs.joc.4c02060 Akash Bisoyi, Amit Behera, Alisha Rani Tripathy, Vijay Kumar Simhadri, Veera Reddy Yatham
|
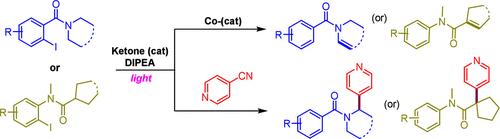
We herein report the chemoselective dehydrogenation and heteroarylation of amides through photoexcited triplet ketone catalysis. Under mild reaction conditions, the generated aryl radical through the halogen atom transfer (XAT) process further undergoes an intramolecular 1,5-HAT event to generate an α-amido alkyl radical, which then intercepted with either cobalt or a reduced cyano arene radical leads to dehydrogenated and heteroarylated products, respectively, in good yields.
中文翻译:

通过光激发三重酮催化实现的自由基易位对酰胺进行化学选择性脱氢和异质芳基化
我们在此报道了通过光激发三联酮催化的酰胺的化学选择性脱氢和杂芳基化。在温和的反应条件下,通过卤素原子转移 (XAT) 过程生成的芳基自由基进一步经历分子内 1,5-HAT 事件,生成 α-酰胺基烷基自由基,然后用钴或还原的氰基芳烃自由基拦截,分别得到脱氢和杂芳基化产物,产率高。
更新日期:2024-11-16
中文翻译:

通过光激发三重酮催化实现的自由基易位对酰胺进行化学选择性脱氢和异质芳基化
我们在此报道了通过光激发三联酮催化的酰胺的化学选择性脱氢和杂芳基化。在温和的反应条件下,通过卤素原子转移 (XAT) 过程生成的芳基自由基进一步经历分子内 1,5-HAT 事件,生成 α-酰胺基烷基自由基,然后用钴或还原的氰基芳烃自由基拦截,分别得到脱氢和杂芳基化产物,产率高。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号