当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diarenofuran[b]-fused BODIPYs: One-Pot SNAr-Suzuki Synthesis and Properties
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.joc.4c02396 Limin He, Na Li, Yanqing Li, Yunxia Zhao, Shulin Gao, Zhaohui Wang, Xiangguang Li, Yanhua Yang, Wei Jiang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.joc.4c02396 Limin He, Na Li, Yanqing Li, Yunxia Zhao, Shulin Gao, Zhaohui Wang, Xiangguang Li, Yanhua Yang, Wei Jiang
|
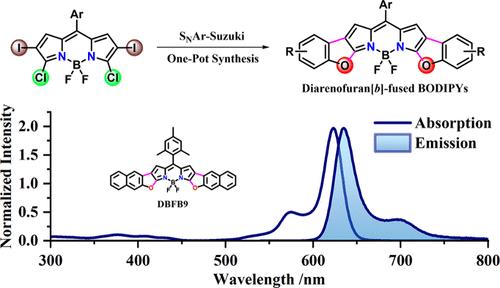
We present a streamlined methodology that integrates regioselective tetrahalogen-BODIPY and o-hydroxyphenylboronic acid in a one-pot process, leveraging SNAr nucleophilic substitution in conjunction with Suzuki coupling to afford diarenofuran [b]-fused BODIPYs (DBFB1–9) with commendable yields (85–95%) and short reaction times (0.5–1.0 h). X-ray structure analyses of DBFB5,7–9 elucidate that these diarenofuran[b]-fused BODIPYs adopt a “butterfly” conformation, maintaining a highly rigid planarity. A comprehensive examination of the spectroscopic and electrochemical properties of these diarenofuran[b]-fused BODIPY derivatives, incorporating various substituents, reveals intriguing characteristics, including pronounced absorption and emission in the near-infrared region (NIR), elevated fluorescence quantum yields (ΦF = 75–89% in dichloromethane), and tunable HOMO–LUMO levels. Remarkably, compared to DBFB1–8, DBFB9 possesses a large extended π-conjugated system, resulting in significant red shifts in its absorption and emission maxima, reaching 623 and 635 nm, respectively, and a reduced HOMO–LUMO gap. Despite this substantial structural expansion, DBFB9 maintains a surprisingly high fluorescence quantum yield (ΦF = 80%), underscoring its exceptional photophysical performance and strong potential for applications requiring efficient NIR emission and robust fluorescence retention.
中文翻译:

Diarenofuran[b] -fused BODIPYs:一锅法 SNAr-Suzuki 合成和性质
我们提出了一种简化的方法,该方法将区域选择性四卤素-BODIPY 和邻羟基苯硼酸整合到一个一锅法中,利用 SNAr 亲核取代与 Suzuki 偶联相结合,得到二烯基呋喃 [b] 融合的 BODIPY (DBFB1-9),产量值得称赞 (85-95%) 和反应时间短 (0.5-1.0 h)。DBFB5,7-9 的 X 射线结构分析阐明,这些淀粉呋喃 [b] 融合的 BODIPY 采用“蝶形”构象,保持高度刚性的平面性。对这些二呋喃[b]融合的 BODIPY 衍生物(包含各种取代基)的光谱和电化学性质进行全面检查,揭示了有趣的特性,包括在近红外区域 (NIR) 中显着的吸收和发射、荧光量子产率升高(ΦF = 75-89% 在二氯甲烷中)和可调的 HOMO-LUMO 水平。值得注意的是,与 DBFB1-8 相比,DBFB9 具有较大的扩展π共轭系统,导致其最大吸收和发射波长发生显著的红移,分别达到 623 和 635 nm,并且 HOMO-LUMO 间隙减小。尽管结构进行了大幅扩展,但 DBFB9 仍保持了令人惊讶的高荧光量子产率 (ΦF = 80%),这凸显了其卓越的光物理性能以及在需要高效 NIR 发射和强大荧光保留能力的应用中的巨大潜力。
更新日期:2024-11-16
中文翻译:

Diarenofuran[b] -fused BODIPYs:一锅法 SNAr-Suzuki 合成和性质
我们提出了一种简化的方法,该方法将区域选择性四卤素-BODIPY 和邻羟基苯硼酸整合到一个一锅法中,利用 SNAr 亲核取代与 Suzuki 偶联相结合,得到二烯基呋喃 [b] 融合的 BODIPY (DBFB1-9),产量值得称赞 (85-95%) 和反应时间短 (0.5-1.0 h)。DBFB5,7-9 的 X 射线结构分析阐明,这些淀粉呋喃 [b] 融合的 BODIPY 采用“蝶形”构象,保持高度刚性的平面性。对这些二呋喃[b]融合的 BODIPY 衍生物(包含各种取代基)的光谱和电化学性质进行全面检查,揭示了有趣的特性,包括在近红外区域 (NIR) 中显着的吸收和发射、荧光量子产率升高(ΦF = 75-89% 在二氯甲烷中)和可调的 HOMO-LUMO 水平。值得注意的是,与 DBFB1-8 相比,DBFB9 具有较大的扩展π共轭系统,导致其最大吸收和发射波长发生显著的红移,分别达到 623 和 635 nm,并且 HOMO-LUMO 间隙减小。尽管结构进行了大幅扩展,但 DBFB9 仍保持了令人惊讶的高荧光量子产率 (ΦF = 80%),这凸显了其卓越的光物理性能以及在需要高效 NIR 发射和强大荧光保留能力的应用中的巨大潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号