当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Practical and Efficient Approach to Scalable Synthesis of Rucaparib
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.oprd.4c00366 Jinjae Park, Cheol-Hong Cheon
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.oprd.4c00366 Jinjae Park, Cheol-Hong Cheon
|
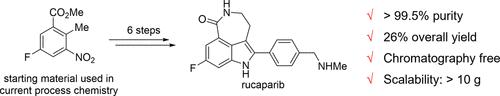
A scalable synthesis of rucaparib was developed from methyl 5-fluoro-2-methyl-3-nitrobenzoate and 4-cyanobenzaldehyde. Methyl 5-fluoro-2-methyl-3-nitrobenzoate was converted into a 2-aminocinnamonitrile derivative, which was subjected to the imino-Stetter reaction with 4-cyanobenzaldehyde to yield trisubstituted indole-3-acetonitrile. The reduction of both nitriles, followed by azepinone scaffold construction and selective monomethylation, completed the synthesis of rucaparib. This synthetic route features the use of inexpensive starting materials, scalability, and ease of purification through recrystallization.
中文翻译:

Rucaparib 可扩展合成的实用且有效的方法
rucaparib 的可扩展合成由 5-氟-2-甲基-3-硝基苯甲酸酯和 4-氰基苯甲醛开发。将 5-氟-2-甲基-3-硝基苯甲酸甲酯转化为 2-氨基肉桂腈衍生物,与 4-氰基苯甲醛进行亚氨基-Stetter 反应,得到三取代的吲哚-3-乙腈。两种腈的还原,然后是氮嗪酮支架构建和选择性单甲基化,完成了 rucaparib 的合成。这种合成路线的特点是使用廉价的起始材料、可扩展性以及易于通过重结晶进行纯化。
更新日期:2024-11-16
中文翻译:

Rucaparib 可扩展合成的实用且有效的方法
rucaparib 的可扩展合成由 5-氟-2-甲基-3-硝基苯甲酸酯和 4-氰基苯甲醛开发。将 5-氟-2-甲基-3-硝基苯甲酸甲酯转化为 2-氨基肉桂腈衍生物,与 4-氰基苯甲醛进行亚氨基-Stetter 反应,得到三取代的吲哚-3-乙腈。两种腈的还原,然后是氮嗪酮支架构建和选择性单甲基化,完成了 rucaparib 的合成。这种合成路线的特点是使用廉价的起始材料、可扩展性以及易于通过重结晶进行纯化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号