当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
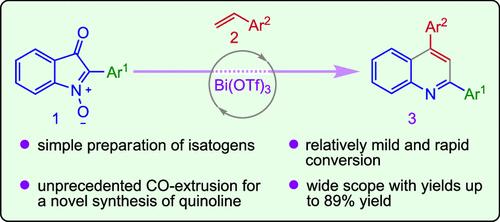
Bi(OTf)3-Catalyzed Cascade Cycloaddition–Decarbonylation–N-Deoxygenative Aromatization between 2-Arylisatogens and Styrenes: Unveiling a Synthesis of 2,4-Diarylquinolines
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03012 Kunlayanee Punjajom, Sukit Chonradeenitchakul, Jumreang Tummatorn, Somsak Ruchirawat, Charnsak Thongsornkleeb
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03012 Kunlayanee Punjajom, Sukit Chonradeenitchakul, Jumreang Tummatorn, Somsak Ruchirawat, Charnsak Thongsornkleeb
|
An unprecedented Bi(OTf)3-catalyzed reaction between 2-arylisatogens and vinylarenes to yield 2,4-diarylquinolines is reported. The transformation occurred via a BiIII-coordination to the embedded anionic nitrone oxygen, which induced a regiospecific stepwise formal [4 + 2]-cycloaddition with vinylarenes to yield a strained tricyclic intermediate, which subsequently extruded gaseous carbon monoxide. N-Deoxygenative aromatization ensued to produce 2,4-diarylquinolines. The protocol was applicable to a variety of substrates to produce the quinolines in up to an 89% yield.
中文翻译:

Bi(OTf)3-催化的 2-芳基生成剂和苯乙烯之间的级联环加成-脱羰-N-脱氧芳构化:揭示 2,4-二芳基喹啉的合成
据报道,2-芳基生成剂和乙烯基芳烃之间发生了前所未有的 Bi(OTf)3 催化反应,生成 2,4-二芳基喹啉。通过对嵌入的阴离子硝氧的 BiIII 配位发生转变,这诱导了与乙烯基芳烃的区域特异性逐步形式 [4 + 2] -环加成反应,以产生应变的三环中间体,随后挤出气态一氧化碳。随后 N-脱氧芳构化生成 2,4-二芳基喹啉。该方案适用于各种底物,以高达 89% 的产率生产喹啉。
更新日期:2024-11-16
中文翻译:

Bi(OTf)3-催化的 2-芳基生成剂和苯乙烯之间的级联环加成-脱羰-N-脱氧芳构化:揭示 2,4-二芳基喹啉的合成
据报道,2-芳基生成剂和乙烯基芳烃之间发生了前所未有的 Bi(OTf)3 催化反应,生成 2,4-二芳基喹啉。通过对嵌入的阴离子硝氧的 BiIII 配位发生转变,这诱导了与乙烯基芳烃的区域特异性逐步形式 [4 + 2] -环加成反应,以产生应变的三环中间体,随后挤出气态一氧化碳。随后 N-脱氧芳构化生成 2,4-二芳基喹啉。该方案适用于各种底物,以高达 89% 的产率生产喹啉。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号