当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Synthesis of (E)- and (Z)-Alkenones via Photoredox C(sp3)–H Alkenylation–Dehydrogenation of o-Iodoarylalkanols with Alkynes
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03707 Liang Zeng, Yin Zhang, Ming Hu, De-Liang He, Xuan-Hui Ouyang, Jin-Heng Li
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03707 Liang Zeng, Yin Zhang, Ming Hu, De-Liang He, Xuan-Hui Ouyang, Jin-Heng Li
|
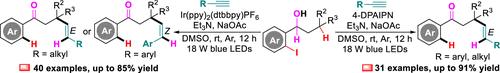
A photoredox C(sp3)–H alkenylation–dehydrogenation of o-iodoarylalkanols with terminal alkynes for the synthesis of (E)- and (Z)-quaternary carbon center-containing pent-4-en-1-ones is described. The stereoselectivity depends on the utilization of alkynes and photocatalysts. While using an organic photocatalyst like 4-DPAIPN manipulates the C(sp3)–H alkenylation–dehydrogenation of o-iodoarylalkanols with arylalkynes to assemble (E)-pent-4-en-1-ones, in the case of an Ir potocatalyst such as Ir(ppy)2(dtbbpy)PF6 the reaction with arylalkynes delivers (Z)-pent-4-en-1-ones. For alkylalkynes, the reaction furnishes (E)-pent-4-en-1-ones exclusively in the presence of 4-DPAIPN or Ir(ppy)2(dtbbpy)PF6.
中文翻译:

通过光氧化还原 C(sp3)-H 烯基化-邻碘芳基烷醇与炔烃脱氢反应对 (E)- 和 (Z)-烯酮的发散合成
描述了邻碘芳基烷醇与末端炔烃的光氧化还原 C(sp3)-H 烯基化-脱氢,用于合成 (E)- 和 (Z)-季碳中心含有 pent-4-en-1-one。立体选择性取决于炔烃和光催化剂的利用率。虽然使用像 4-DPAIPN 这样的有机光催化剂操纵邻碘芳基烷醇与芳基烷基的 C(sp3)-H 烯基化-脱氢以组装 (E)-pent-4-en-1-one,但在 Ir Potocatalyst 如 Ir(ppy)2(dtbbpy)PF6 的情况下,与芳基烷烃的反应产生 (Z)-pent-4-en-1-ones。对于烷基炔烃,反应仅在 4-DPAIPN 或 Ir(ppy)2(dtbbpy)PF6 存在下提供 (E)-pent-4-en-1-one。
更新日期:2024-11-16
中文翻译:

通过光氧化还原 C(sp3)-H 烯基化-邻碘芳基烷醇与炔烃脱氢反应对 (E)- 和 (Z)-烯酮的发散合成
描述了邻碘芳基烷醇与末端炔烃的光氧化还原 C(sp3)-H 烯基化-脱氢,用于合成 (E)- 和 (Z)-季碳中心含有 pent-4-en-1-one。立体选择性取决于炔烃和光催化剂的利用率。虽然使用像 4-DPAIPN 这样的有机光催化剂操纵邻碘芳基烷醇与芳基烷基的 C(sp3)-H 烯基化-脱氢以组装 (E)-pent-4-en-1-one,但在 Ir Potocatalyst 如 Ir(ppy)2(dtbbpy)PF6 的情况下,与芳基烷烃的反应产生 (Z)-pent-4-en-1-ones。对于烷基炔烃,反应仅在 4-DPAIPN 或 Ir(ppy)2(dtbbpy)PF6 存在下提供 (E)-pent-4-en-1-one。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号