当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Pyridone-Enhanced Mn-Catalysis for the Synthesis of ortho-Deuterated Aromatic Nitriles
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c04061 Yanran Liu, Tao Qian, Jian-Fei Bai, Jinfeng Zheng, You Zhou, Zhi-Jiang Jiang, Jia Chen, Zhanghua Gao
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c04061 Yanran Liu, Tao Qian, Jian-Fei Bai, Jinfeng Zheng, You Zhou, Zhi-Jiang Jiang, Jia Chen, Zhanghua Gao
|
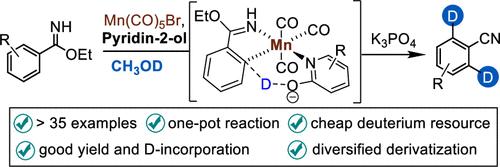
We herein present a method for the synthesis of ortho-deuterated aryl nitrile using Mn(CO)5Br as catalyst with CH3OD as deuteratium source, where the structure of aryl imidates is used for interconversion with a cyanide group. This method features a broad substrate range and excellent functional group tolerance, allowing late modification of various complex molecules with good yields and deuterium incorporation. Mechanistic studies suggest that 2-pyridone is crucial to the success of this chemistry, serving as an endogenous base that enhances the rate of hydrogen isotope exchange.
中文翻译:

2-吡啶酮增强 Mn-催化合成邻位氘代芳香腈
我们在此提出了一种以 Mn(CO)5Br 为催化剂,以 CH3OD 为氘源合成邻氘芳基腈的方法,其中芳基亚胺酸盐的结构用于与氰化物基团相互转化。该方法具有广泛的底物范围和出色的官能团耐受性,允许对各种复杂分子进行后期修饰,具有良好的产率和氘掺入。机理研究表明,2-吡啶酮对这种化学的成功至关重要,它是提高氢同位素交换速率的内源性碱基。
更新日期:2024-11-16
中文翻译:

2-吡啶酮增强 Mn-催化合成邻位氘代芳香腈
我们在此提出了一种以 Mn(CO)5Br 为催化剂,以 CH3OD 为氘源合成邻氘芳基腈的方法,其中芳基亚胺酸盐的结构用于与氰化物基团相互转化。该方法具有广泛的底物范围和出色的官能团耐受性,允许对各种复杂分子进行后期修饰,具有良好的产率和氘掺入。机理研究表明,2-吡啶酮对这种化学的成功至关重要,它是提高氢同位素交换速率的内源性碱基。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号