Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimizing Cu 3d Bands with Nanotubular SnO2 to Boost Their Catalytic Transfer Hydrogenation Activity
Langmuir ( IF 3.7 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.langmuir.4c03318 Yu Pan, Rongjie Cai, Zening Li, Yuan Lin, Yunyun Gui, Lijun Liu
Langmuir ( IF 3.7 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.langmuir.4c03318 Yu Pan, Rongjie Cai, Zening Li, Yuan Lin, Yunyun Gui, Lijun Liu
|
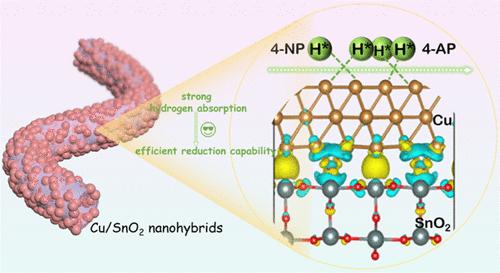
Catalytic transfer hydrogenation (CTH) using Cu nanocatalysts offers significant advantages over direct high-pressure hydrogenation. However, the active hydrogen (H*) in this process exhibits poor adsorption and tends to release H2 readily due to the fully occupied 3d states of Cu. To address this issue, a tubular SnO2 support with electron-accepting ability was selected to host Cu nanoparticles, aiming to optimize the Cu 3d bands. The Cu/SnO2 nanohybrids were prepared through an electrospinning technique, followed by hydrothermal synthesis. As evidenced by X-ray photoelectron spectroscopy (XPS) binding energy shifts and density functional theory (DFT) simulations, some electrons from Cu transferred to SnO2 in the Cu/SnO2 nanohybrids due to their different work functions. Such electron transfer enables the Cu 3d orbitals to lose electrons and alters its valence configuration from 3d10 to 3d10–x, which enhances the adsorption of active H* atoms and thereby inhibits undesirable H2 release. The 15 wt % Cu/SnO2 exhibits improved catalytic hydrogenation of 4-nitrophenol with NaBH4, with an optimal normalized rate constant of 56.98 mg–1 min–1 and a turnover frequency of 4.82 min–1, surpassing most reported catalysts. The enhanced activity is attributed to the optimized electronic states, improved hydrogen adsorption, and the tubular structure of the support. This work might shed light on developing more non-noble metal nanocatalysts for CTH by tuning their d bands with appropriate oxide supports.
中文翻译:

使用纳米管 SnO2 优化 Cu 3d 波段以提高其催化转移加氢活性
与直接高压加氢相比,使用 Cu 纳米催化剂的催化转移加氢 (CTH) 具有显著优势。然而,该过程中的活性氢 (H*) 表现出较差的吸附性,并且由于 Cu 的完全占据 3d 状态,往往很容易释放 H2。为了解决这个问题,选择了一种具有电子接受能力的管状 SnO2 载体来承载 Cu 纳米颗粒,旨在优化 Cu 3d 波段。Cu/SnO2 纳米杂化物是通过静电纺丝技术制备的,然后是水热合成。正如 X 射线光电子能谱 (XPS) 结合能转移和密度泛函理论 (DFT) 模拟所证明的那样,由于 Cu/SnO 2 纳米杂化物中的一些电子由于功函数不同而转移到 Cu/SnO2 纳米杂化物中的 SnO2。这种电子转移使 Cu 3d 轨道失去电子,并将其价构型从 3d10 改变为 3d10-x,从而增强活性 H* 原子的吸附,从而抑制不需要的 H2 释放。15 wt % Cu/SnO2 表现出改进的 4-硝基苯酚与 NaBH4 的催化加氢反应,最佳归一化速率常数为 56.98 mg–1 min–1,周转频率为 4.82 min–1,超过了大多数已报道的催化剂。增强的活性归因于优化的电子态、改进的氢吸附和载体的管状结构。这项工作可能通过用适当的氧化物载体调整 CTH 的 d 带来开发更多用于 CTH 的非贵金属纳米催化剂。
更新日期:2024-11-16
中文翻译:

使用纳米管 SnO2 优化 Cu 3d 波段以提高其催化转移加氢活性
与直接高压加氢相比,使用 Cu 纳米催化剂的催化转移加氢 (CTH) 具有显著优势。然而,该过程中的活性氢 (H*) 表现出较差的吸附性,并且由于 Cu 的完全占据 3d 状态,往往很容易释放 H2。为了解决这个问题,选择了一种具有电子接受能力的管状 SnO2 载体来承载 Cu 纳米颗粒,旨在优化 Cu 3d 波段。Cu/SnO2 纳米杂化物是通过静电纺丝技术制备的,然后是水热合成。正如 X 射线光电子能谱 (XPS) 结合能转移和密度泛函理论 (DFT) 模拟所证明的那样,由于 Cu/SnO 2 纳米杂化物中的一些电子由于功函数不同而转移到 Cu/SnO2 纳米杂化物中的 SnO2。这种电子转移使 Cu 3d 轨道失去电子,并将其价构型从 3d10 改变为 3d10-x,从而增强活性 H* 原子的吸附,从而抑制不需要的 H2 释放。15 wt % Cu/SnO2 表现出改进的 4-硝基苯酚与 NaBH4 的催化加氢反应,最佳归一化速率常数为 56.98 mg–1 min–1,周转频率为 4.82 min–1,超过了大多数已报道的催化剂。增强的活性归因于优化的电子态、改进的氢吸附和载体的管状结构。这项工作可能通过用适当的氧化物载体调整 CTH 的 d 带来开发更多用于 CTH 的非贵金属纳米催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号