Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-15 , DOI: 10.1002/adsc.202401220 Juting Liao, Ruirui Zhai, Xiaoyang Gao, Hongliang Wu, Dulin Kong, Shuojin Wang, Xun Chen
|
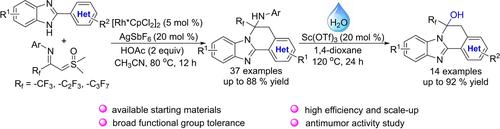
An efficient Rh(III)-catalyzed C−H bond activation/cyclization of 2-arylbenzimidazoles with CF3-imidoyl sulfoxonium ylides has been achieved, yielding diverse CF3− and amino-disubstituted 5,6-dihydrobenzoimidazo[2,1-a]isoquinolines, which could undergo a deaminative hydroxylation to access CF3− and hydroxy-disubstituted 5,6-dihydrobenzoimidazo[2,1-a]isoquinolines catalyzed by Sc(OTf)3. This developed strategy features easily available starting materials, broad substrate scope, good scalability and high efficiency. Moreover, the antitumor activities of selected products against some human cancer cell lines were also investigated, and the results indicated that several products show antiproliferative activities.
中文翻译:

Rh(III) 催化的 2-芳基苯并咪唑与 CF3-亚胺酰亚胺铵的 C-H 环化反应和进一步的 Sc(III) 催化的脱酰胺羟基化反应
已经实现了 2-芳基苯并咪唑与 CF3-亚胺酰亚甲基亚甲基酰亚胺酰亚胺酰化物的高效 Rh(III) 催化的 C-H 键活化/环化,产生了多种 CF3− 和氨基二取代的 5,6-二氢苯并咪唑[2,1-a] 异喹啉,其可以经历脱酰胺羟基化以获得 CF3- 和羟基二取代的 5,6-二氢苯并咪唑[2,1-a] 异喹啉由 Sc(OTf)3 催化.这种开发的策略具有易于获得起始材料、广泛的基材范围、良好的可扩展性和高效率。此外,还研究了所选产品对一些人类癌细胞系的抗肿瘤活性,结果表明几种产品显示出抗增殖活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号