Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge Separation-Engineered Piezoelectric Ultrathin Nanorods Modulate Tumor Stromal Microenvironment and Enhance Cell Immunogenicity for Synergistically Piezo-Thermal-Immune Therapy
Small ( IF 13.0 ) Pub Date : 2024-11-16 , DOI: 10.1002/smll.202408038 Qian Wang, Jun Du, Fujun Yang, Sijia Wu, Luna Zhu, Xueyu Li, Han Yang, Yuqing Miao, Yuhao Li
Small ( IF 13.0 ) Pub Date : 2024-11-16 , DOI: 10.1002/smll.202408038 Qian Wang, Jun Du, Fujun Yang, Sijia Wu, Luna Zhu, Xueyu Li, Han Yang, Yuqing Miao, Yuhao Li
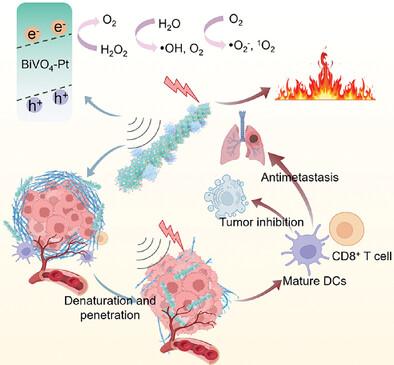
|
The tumor microenvironment (TME) is characterized by hypoxia and low immunogenicity, with a dense and rigid extracellular matrix (ECM) that impedes the diffusion of therapeutic agents and immune cells, thereby limiting the efficacy of immunotherapy. To overcome these challenges, an oxygen defect piezoelectric-photothermal sensitizer, bismuth vanadate nanorod-supported platinum nanodots (BVP) is developed. The integration of platinum enhances the photothermal effect and improves charge separation efficiency under ultrasound, leading to increased heat generation and the production of reactive oxygen species (ROS) and oxygen. Platinum also catalyzes the conversion of hydrogen peroxide in the TME to oxygen, which serves as both a ROS source and a means to alleviate tumor hypoxia, thereby reversing the immunosuppressive TME. Moreover, the coordination of bismuth ions with glutathione further amplifies cellular oxidative stress. The generated heat and ROS not only denature the collagen in the ECM, facilitating the deeper penetration of BVP into the tumor but also induce immunogenic cell death in tumor cells. Through the “degeneration and penetration” strategy, photoacoustic therapy effectively activates immune cells, inhibiting both tumor growth and metastasis. This study introduces a pioneering approach in the design of antitumor nanomedicines aimed at reversing the immunosuppressive characteristics of the TME.
中文翻译:

电荷分离工程压电超薄纳米棒可调节肿瘤基质微环境并增强细胞免疫原性,以实现协同压电-热-免疫疗法
肿瘤微环境 (TME) 的特点是缺氧和低免疫原性,具有致密而坚硬的细胞外基质 (ECM),阻碍了治疗剂和免疫细胞的扩散,从而限制了免疫治疗的疗效。为了克服这些挑战,开发了一种氧缺陷压电-光热敏化剂,钒酸铋纳米棒负载的铂纳米点 (BVP)。铂的整合增强了光热效应,提高了超声下的电荷分离效率,导致热量产生增加,并产生了活性氧 (ROS) 和氧气。铂还催化 TME 中的过氧化氢转化为氧气,氧气既是 ROS 来源,也是缓解肿瘤缺氧的手段,从而逆转免疫抑制性 TME。此外,铋离子与谷胱甘肽的配位进一步放大了细胞氧化应激。产生的热量和 ROS 不仅使 ECM 中的胶原蛋白变性,促进 BVP 更深入地渗透到肿瘤中,而且还诱导肿瘤细胞中的免疫原性细胞死亡。通过“变性和渗透性”策略,光声疗法有效地激活免疫细胞,抑制肿瘤生长和转移。本研究介绍了一种开创性的抗肿瘤纳米药物设计方法,旨在逆转 TME 的免疫抑制特性。
更新日期:2024-11-16
中文翻译:

电荷分离工程压电超薄纳米棒可调节肿瘤基质微环境并增强细胞免疫原性,以实现协同压电-热-免疫疗法
肿瘤微环境 (TME) 的特点是缺氧和低免疫原性,具有致密而坚硬的细胞外基质 (ECM),阻碍了治疗剂和免疫细胞的扩散,从而限制了免疫治疗的疗效。为了克服这些挑战,开发了一种氧缺陷压电-光热敏化剂,钒酸铋纳米棒负载的铂纳米点 (BVP)。铂的整合增强了光热效应,提高了超声下的电荷分离效率,导致热量产生增加,并产生了活性氧 (ROS) 和氧气。铂还催化 TME 中的过氧化氢转化为氧气,氧气既是 ROS 来源,也是缓解肿瘤缺氧的手段,从而逆转免疫抑制性 TME。此外,铋离子与谷胱甘肽的配位进一步放大了细胞氧化应激。产生的热量和 ROS 不仅使 ECM 中的胶原蛋白变性,促进 BVP 更深入地渗透到肿瘤中,而且还诱导肿瘤细胞中的免疫原性细胞死亡。通过“变性和渗透性”策略,光声疗法有效地激活免疫细胞,抑制肿瘤生长和转移。本研究介绍了一种开创性的抗肿瘤纳米药物设计方法,旨在逆转 TME 的免疫抑制特性。































 京公网安备 11010802027423号
京公网安备 11010802027423号