Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the mechanistic ‘Black Box’ of heterogeneous condensation reactions catalyzed by aminosilicas
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.jcat.2024.115852 Parijat Borah, Preeti Nanda Sahu, Anik Sen, Miquel A. Pericàs
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-16 , DOI: 10.1016/j.jcat.2024.115852 Parijat Borah, Preeti Nanda Sahu, Anik Sen, Miquel A. Pericàs
|
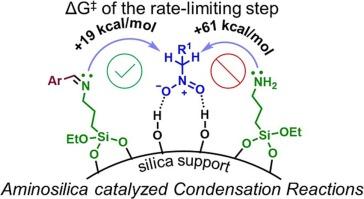
Primary amino-functionalized silicas (H2 N-SiO2 ) are well known acid-base cooperative catalysts for many organic transformations, including carbon–carbon (C–C) bond forming condensation reactions, and much attention has been devoted to the elucidation of their action mode. However, to our surprise, the mechanism of Henry reactions and Knoevenagel condensations catalyzed by H2 N-SiO2 is still paradoxical, and the identity of the actual base species, transition states, reactivity, and product selectivity, remain as debatable topics of discussion. Herein we propose a brand-new reaction mechanism for H2 N-SiO2 -catalyzed Henry reactions that overcomes all prior inconsistencies. With the aid of Hammett analysis and density functional theory (DFT) calculations, we have effectively identified several critical transition states and are able to explain reactivity and product selectivity. This study revealed that H2 N-SiO2 catalyzed Henry reactions of aldehydes and nitro compounds follow the imine mechanism to afford olefin adducts as only possible products. In addition, we utilized our findings to comprehend the mechanism of Knoevenagel condensation, a comparable reaction, dispelling a more than two-decade old misconception regarding the nature of the active base involved.
中文翻译:

揭示氨基二氧化硅催化的非均相缩合反应的机理“黑匣子”
伯氨基官能化二氧化硅 (H2N-SiO2) 是许多有机转化的众所周知的酸碱协同催化剂,包括碳-碳 (C-C) 键形成缩合反应,人们已经非常关注阐明它们的作用模式。然而,令我们惊讶的是,H2N-SiO2 催化的 Henry 反应和 Knoevenagel 缩合的机制仍然是自相矛盾的,实际碱基种类、过渡态、反应性和产物选择性的身份仍然是有争议的讨论话题。在此,我们提出了一种全新的 H2N-SiO2 催化亨利反应反应机理,克服了所有先前的不一致。在 Hammett 分析和密度泛函理论 (DFT) 计算的帮助下,我们有效地确定了几种关键的过渡态,并能够解释反应性和产物选择性。这项研究表明,H2N-SiO2 催化醛和硝基化合物的亨利反应遵循亚胺机制,使烯烃加合物成为唯一可能的产物。此外,我们利用我们的发现来理解 Knoevenagel 缩合的机制,这是一种类似的反应,消除了二十多年来关于所涉及的活性碱的性质的误解。
更新日期:2024-11-16
中文翻译:

揭示氨基二氧化硅催化的非均相缩合反应的机理“黑匣子”
伯氨基官能化二氧化硅 (H2N-SiO2) 是许多有机转化的众所周知的酸碱协同催化剂,包括碳-碳 (C-C) 键形成缩合反应,人们已经非常关注阐明它们的作用模式。然而,令我们惊讶的是,H2N-SiO2 催化的 Henry 反应和 Knoevenagel 缩合的机制仍然是自相矛盾的,实际碱基种类、过渡态、反应性和产物选择性的身份仍然是有争议的讨论话题。在此,我们提出了一种全新的 H2N-SiO2 催化亨利反应反应机理,克服了所有先前的不一致。在 Hammett 分析和密度泛函理论 (DFT) 计算的帮助下,我们有效地确定了几种关键的过渡态,并能够解释反应性和产物选择性。这项研究表明,H2N-SiO2 催化醛和硝基化合物的亨利反应遵循亚胺机制,使烯烃加合物成为唯一可能的产物。此外,我们利用我们的发现来理解 Knoevenagel 缩合的机制,这是一种类似的反应,消除了二十多年来关于所涉及的活性碱的性质的误解。































 京公网安备 11010802027423号
京公网安备 11010802027423号