当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gut commensal bacteria Parabacteroides goldsteinii-derived outer membrane vesicles suppress skin inflammation in psoriasis
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.jconrel.2024.11.014 Dandan Su, Manchun Li, Yuedong Xie, Zhanxue Xu, Guowen Lv, Yaming Jiu, Jingxiong Lin, Chih-Jung Chang, Hongbo Chen, Fang Cheng
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-18 , DOI: 10.1016/j.jconrel.2024.11.014 Dandan Su, Manchun Li, Yuedong Xie, Zhanxue Xu, Guowen Lv, Yaming Jiu, Jingxiong Lin, Chih-Jung Chang, Hongbo Chen, Fang Cheng
|
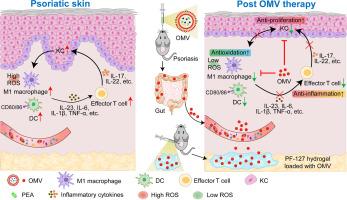
Despite gut microbiota-derived extracellular vesicles (EVs) serving as pivotal mediators in bacteria-host cell interactions, their potential role in modulating skin inflammation remains poorly understood. Here, we developed strategies for mass production of Parabacteroides goldsteinii -derived outer membrane vesicles (Pg OMVs), commonly known as EVs. We found that orally administered Pg OMVs can reach the colon, traverse the intestinal barrier, and circulate to the inflamed skin of psoriasis-like mice, resulting in reduced epidermal hyperplasia, suppressed infiltration of inflammatory cells in the skin lesions, and effective amelioration of both skin and systemic inflammation. Additionally, subcutaneous injection of thermosensitive PF-127 hydrogel loaded with Pg OMVs exerts similar immunomodulatory effects, allowing sustained release of Pg OMVs into skin cells, effectively suppressing skin inflammation and ameliorating symptoms of psoriasis. This study unveils the importance of gut microbiota-derived OMVs, which can target inflamed skin via both the gut-skin axis and local skin administration, providing a promising alternative to live bacteria therapy for the treatment of skin inflammatory diseases.
中文翻译:

肠道共生菌 副拟杆菌 goldsteinii 衍生的外膜囊泡抑制银屑病中的皮肤炎症
尽管肠道微生物群衍生的细胞外囊泡 (EV) 是细菌-宿主细胞相互作用的关键介质,但它们在调节皮肤炎症方面的潜在作用仍然知之甚少。在这里,我们开发了大规模生产 Parabacteroides goldsteinii 衍生的外膜囊泡 (Pg OMV),通常称为 EV 的策略。我们发现口服 Pg OMVs 可以到达结肠,穿过肠道屏障,并循环到银屑病样小鼠发炎的皮肤,从而减少表皮增生,抑制炎症细胞在皮肤病变中的浸润,并有效改善皮肤和全身炎症。此外,皮下注射载有 Pg OMV 的热敏 PF-127 水凝胶具有类似的免疫调节作用,允许 Pg OMV 持续释放到皮肤细胞中,有效抑制皮肤炎症并改善银屑病症状。本研究揭示了肠道微生物群衍生的 OMV 的重要性,它可以通过肠道-皮肤轴和局部皮肤给药针对发炎的皮肤,为治疗皮肤炎症性疾病提供了一种有前途的活细菌疗法替代方案。
更新日期:2024-11-18
中文翻译:

肠道共生菌 副拟杆菌 goldsteinii 衍生的外膜囊泡抑制银屑病中的皮肤炎症
尽管肠道微生物群衍生的细胞外囊泡 (EV) 是细菌-宿主细胞相互作用的关键介质,但它们在调节皮肤炎症方面的潜在作用仍然知之甚少。在这里,我们开发了大规模生产 Parabacteroides goldsteinii 衍生的外膜囊泡 (Pg OMV),通常称为 EV 的策略。我们发现口服 Pg OMVs 可以到达结肠,穿过肠道屏障,并循环到银屑病样小鼠发炎的皮肤,从而减少表皮增生,抑制炎症细胞在皮肤病变中的浸润,并有效改善皮肤和全身炎症。此外,皮下注射载有 Pg OMV 的热敏 PF-127 水凝胶具有类似的免疫调节作用,允许 Pg OMV 持续释放到皮肤细胞中,有效抑制皮肤炎症并改善银屑病症状。本研究揭示了肠道微生物群衍生的 OMV 的重要性,它可以通过肠道-皮肤轴和局部皮肤给药针对发炎的皮肤,为治疗皮肤炎症性疾病提供了一种有前途的活细菌疗法替代方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号