当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strategic delivery of rapamycin and ranibizumab with intravitreal hydrogel depot disrupts multipathway-driven angiogenesis loop for boosted wAMD therapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.jconrel.2024.11.011 Xi Jiang, Congyan Liu, Qun Zhang, Yanli Lv, Chen Lu, Wenting Su, Jing Zhou, Huangqin Zhang, Huiling Gong, Yuping Liu, Songtao Yuan, Yan Chen, Ding Qu
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-11-21 , DOI: 10.1016/j.jconrel.2024.11.011 Xi Jiang, Congyan Liu, Qun Zhang, Yanli Lv, Chen Lu, Wenting Su, Jing Zhou, Huangqin Zhang, Huiling Gong, Yuping Liu, Songtao Yuan, Yan Chen, Ding Qu
|
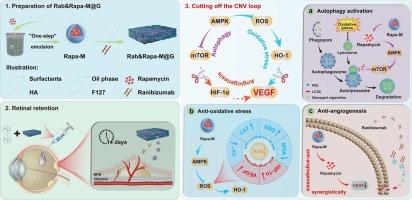
Autophagic dysfunction-induced deterioration of the retinal microenvironment drives the progression of wet age-related macular degeneration (wAMD). The efficacy of single-target anti-VEGF antibodies in treating wAMD has long been suboptimal due to the intricate interplay between autophagy dysfunction, oxidative stress, and angiogenesis. Here, we introduce an intravitreal hydrogel depot, named Rab&Rapa-M@G, consisting of rapamycin-loaded microemulsion (Rapa-M, an mTOR inhibitor), ranibizumab (anti-VEGF antibody), and a thermosensitive hydrogel matrix. A single intravitreal injection of Rab&Rapa-M@G can sustainably deliver Rapa-M and ranibizumab to the retinal pigment epithelium for at least 14 days. This formulation significantly improves retinal autophagic flux homeostasis and reduces oxidative stress injury in wAMD mice by modulating the AMPK/mTOR/HIF-1α/VEGF and AMPK/ROS/HO-1/VEGF pathways. Consequently, it synergistically disrupts the “autophagic dysfunction-oxidative stress-angiogenesis” loop, leading to a remarkable reduction in choroidal neovascularization area and retinal damage compared to ranibizumab alone. Notably, the sequential administration of ranibizumab and Rab&Rapa-M@G further enhances the overall anti-wAMD efficacy, achieved through sequential delivery of Rab and Rapa, allowing for a more precise grasp of the treatment window. In conclusion, this hydrogel depot design, with its sequential and sustained delivery of mTOR inhibitors and anti-VEGF antibodies, offers a promising strategy for multi-target synergistic therapy in wAMD.
中文翻译:

雷帕霉素和雷珠单抗与玻璃体内水凝胶长效制剂的战略性递送破坏了增强 wAMD 治疗的多途径驱动的血管生成循环
自噬功能障碍诱导的视网膜微环境恶化推动了湿性年龄相关性黄斑变性 (wAMD) 的进展。由于自噬功能障碍、氧化应激和血管生成之间错综复杂的相互作用,单靶点抗 VEGF 抗体治疗 wAMD 的疗效长期以来一直不理想。在这里,我们介绍了一种名为 Rab&Rapa-M@G 的玻璃体内水凝胶库,由负载雷帕霉素的微乳剂(Rapa-M,一种 mTOR 抑制剂)、雷珠单抗(抗 VEGF 抗体)和热敏性水凝胶基质组成。单次玻璃体内注射 Rab&Rapa-M@G 可以可持续地将 Rapa-M 和雷珠单抗输送到视网膜色素上皮至少 14 天。该制剂通过调节 AMPK/mTOR/HIF-1α/VEGF 和 AMPK/ROS/HO-1/VEGF 通路,显著改善视网膜自噬通量稳态并减少 wAMD 小鼠的氧化应激损伤。因此,它协同破坏“自噬功能障碍-氧化应激-血管生成”循环,与单独使用雷珠单抗相比,导致脉络膜新生血管区域和视网膜损伤显着减少。值得注意的是,雷珠单抗和 Rab&Rapa-M@G 的序贯给药进一步增强了整体抗 wAMD 疗效,这是通过序贯给药 Rab 和 Rapa 实现的,从而可以更精确地掌握治疗窗口。总之,这种水凝胶长效库设计,凭借其 mTOR 抑制剂和抗 VEGF 抗体的连续和持续递送,为 wAMD 中的多靶点协同治疗提供了一种有前途的策略。
更新日期:2024-11-21
中文翻译:

雷帕霉素和雷珠单抗与玻璃体内水凝胶长效制剂的战略性递送破坏了增强 wAMD 治疗的多途径驱动的血管生成循环
自噬功能障碍诱导的视网膜微环境恶化推动了湿性年龄相关性黄斑变性 (wAMD) 的进展。由于自噬功能障碍、氧化应激和血管生成之间错综复杂的相互作用,单靶点抗 VEGF 抗体治疗 wAMD 的疗效长期以来一直不理想。在这里,我们介绍了一种名为 Rab&Rapa-M@G 的玻璃体内水凝胶库,由负载雷帕霉素的微乳剂(Rapa-M,一种 mTOR 抑制剂)、雷珠单抗(抗 VEGF 抗体)和热敏性水凝胶基质组成。单次玻璃体内注射 Rab&Rapa-M@G 可以可持续地将 Rapa-M 和雷珠单抗输送到视网膜色素上皮至少 14 天。该制剂通过调节 AMPK/mTOR/HIF-1α/VEGF 和 AMPK/ROS/HO-1/VEGF 通路,显著改善视网膜自噬通量稳态并减少 wAMD 小鼠的氧化应激损伤。因此,它协同破坏“自噬功能障碍-氧化应激-血管生成”循环,与单独使用雷珠单抗相比,导致脉络膜新生血管区域和视网膜损伤显着减少。值得注意的是,雷珠单抗和 Rab&Rapa-M@G 的序贯给药进一步增强了整体抗 wAMD 疗效,这是通过序贯给药 Rab 和 Rapa 实现的,从而可以更精确地掌握治疗窗口。总之,这种水凝胶长效库设计,凭借其 mTOR 抑制剂和抗 VEGF 抗体的连续和持续递送,为 wAMD 中的多靶点协同治疗提供了一种有前途的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号