当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Vinylogous Michael/Oxa-Michael Tandem Reaction between β,γ-Unsaturated Amides and Isatin-Derived β,γ-Unsaturated α-Ketoesters
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.joc.4c02066 Shan-Shan Xu, Zi-Yu Li, Meng-Yu Liu, Feng Sha, Xin-Yan Wu
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.joc.4c02066 Shan-Shan Xu, Zi-Yu Li, Meng-Yu Liu, Feng Sha, Xin-Yan Wu
|
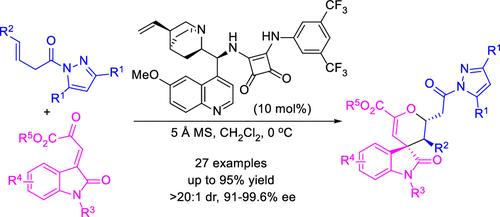
An organocatalytic asymmetric vinylogous Michael/oxa-Michael tandem reaction between β,γ-unsaturated pyrazoleamides and isatin-derived β,γ-unsaturated ketoesters has been developed with excellent regio-, diastereo-, and enantioselectivities. The methodology provides an effective approach to construct enantiomerically pure 3,4′-pyranyl spirooxindole derivatives containing three contiguous chiral centers. Moreover, the transformations of the chiral products, including the removal and reduction of the pyrazole group, have been investigated.
中文翻译:

β,γ-不饱和酰胺与阿丁衍生的β,γ-不饱和α酮酯之间的不对称乙烯基 Michael/Oxa-Michael 串联反应
β,γ-不饱和吡唑酰胺和β,γ-不饱和酮酯之间的有机催化不对称乙烯基 Michael/oxa-Michael 串联反应已被开发出来,具有优异的区域、非对映和对映选择性。该方法提供了一种构建对映体纯的 3,4′-吡喃基螺吲哚衍生物的有效方法,其中包含三个连续的手性中心。此外,已经研究了手性产物的转化,包括吡唑基团的去除和还原。
更新日期:2024-11-15
中文翻译:

β,γ-不饱和酰胺与阿丁衍生的β,γ-不饱和α酮酯之间的不对称乙烯基 Michael/Oxa-Michael 串联反应
β,γ-不饱和吡唑酰胺和β,γ-不饱和酮酯之间的有机催化不对称乙烯基 Michael/oxa-Michael 串联反应已被开发出来,具有优异的区域、非对映和对映选择性。该方法提供了一种构建对映体纯的 3,4′-吡喃基螺吲哚衍生物的有效方法,其中包含三个连续的手性中心。此外,已经研究了手性产物的转化,包括吡唑基团的去除和还原。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号