当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical and Biochemical Approaches to an Enantiomerically Pure 3,4-Disubstituted Tetrahydrofuran Derivative at a Multikilogram Scale: The Power of KRED
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.oprd.4c00388 Antony Bigot, Alain Rabion, Jean-Bernard Landier, Geoffrey Laronze, Frédéric Petit, Stéphanie Deprets, Jean-Marc Michot, Changxia Yuan, Fenglai Sun, Han Chen, Longlei Hou, Dalin Tang
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.oprd.4c00388 Antony Bigot, Alain Rabion, Jean-Bernard Landier, Geoffrey Laronze, Frédéric Petit, Stéphanie Deprets, Jean-Marc Michot, Changxia Yuan, Fenglai Sun, Han Chen, Longlei Hou, Dalin Tang
|
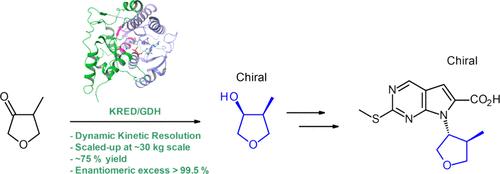
A scalable synthesis of (3S,4S)-4-methyltetrahydrofuran-3-ol involving a keto reductase-mediated enantio- and diastereoselective reduction of a racemic ketone substrate is reported. This chiral intermediate was initially produced using a low-yielding three-step synthesis from ketone, deemed not usable for future batches. Looking for a scalable and environmental process: an eco-design approach led to a one-step, highly enantio- and diastereoselective biocatalytic reduction of the ketone to the targeted intermediate (3S,4S)-4-methyltetrahydrofuran-3-ol. In addition, the reaction operates via dynamic kinetic resolution under unprecedented mild conditions of temperature and pH, allowing for a full conversion of the ketone substrate into the desired enantiomer. The new route led to a significant improvement of all the key performance indicators, including PMI, solvent, and waste.
中文翻译:

千克级对映体纯 3,4-二取代四氢呋喃衍生物的化学和生化方法:KRED 的力量
报道了 (3S,4S)-4-甲基四氢呋喃-3-醇的可扩展合成,涉及酮还原酶介导的外消旋酮底物的对映和非对映选择性还原。这种手性中间体最初是使用低产率的酮三步合成法生产的,被认为不适用于未来的批次。寻找可扩展的环保工艺:一种生态设计方法导致酮的一步、高度对映和非对映选择性生物催化还原为目标中间体 (3S,4 S)-4-甲基四氢呋喃-3-醇。此外,该反应在前所未有的温和温度和 pH 条件下通过动态动力学分辨率进行,从而允许酮底物完全转化为所需的对映异构体。新路线导致所有关键绩效指标的显著改善,包括 PMI、溶剂和废物。
更新日期:2024-11-15
中文翻译:

千克级对映体纯 3,4-二取代四氢呋喃衍生物的化学和生化方法:KRED 的力量
报道了 (3S,4S)-4-甲基四氢呋喃-3-醇的可扩展合成,涉及酮还原酶介导的外消旋酮底物的对映和非对映选择性还原。这种手性中间体最初是使用低产率的酮三步合成法生产的,被认为不适用于未来的批次。寻找可扩展的环保工艺:一种生态设计方法导致酮的一步、高度对映和非对映选择性生物催化还原为目标中间体 (3S,4 S)-4-甲基四氢呋喃-3-醇。此外,该反应在前所未有的温和温度和 pH 条件下通过动态动力学分辨率进行,从而允许酮底物完全转化为所需的对映异构体。新路线导致所有关键绩效指标的显著改善,包括 PMI、溶剂和废物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号