当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
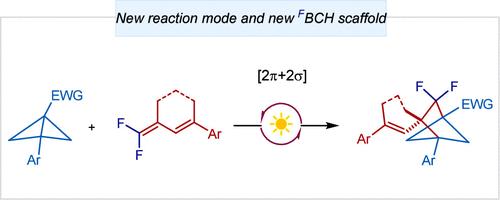
gem-Difluorobicyclo[2.1.1]hexanes via Photochemical [2π + 2σ] Cycloaddition Initiated by Oxidative Activation of gem-Difluorodienes
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03798 Zhenda Fu, Jianzheng Cheng, Xiao-Xi Li, Xingwei Li, Songjie Yu
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-15 , DOI: 10.1021/acs.orglett.4c03798 Zhenda Fu, Jianzheng Cheng, Xiao-Xi Li, Xingwei Li, Songjie Yu
|
The incorporation of fluorine atoms into three-dimensional sp3-rich scaffolds represents an attractive tactic during bioisosteric evolution campaigns by endowing bioisosteric candidates with improved pharmacokinetic properties. Photo- or Lewis acid-mediated bicyclo[1.1.0]butane cycloaddition has offered an efficient approach for the construction of numerous regular bicyclo[n.1.1] scaffolds (n = 1–5) but remains a significant challenge to the synthesis of related 3D fluorinated scaffolds. Herein, we unveiled a photochemical single-electron oxidative strategy for gem-difluorodiene activation and subsequent [2π + 2σ] cycloaddition with bicyclo[1.1.0]butanes to provide a broad range of gem-difluorobicyclo[2.1.1]hexane scaffolds containing several post-transformable handles. A combination of experimental and computational mechanistic studies suggested that the conjugated π system of gem-difluorodiene plays important dual roles in promoting its preferential single-electron oxidation and stabilizing various radical-involved intermediates during the cyclization.
中文翻译:

gem-二氟双环[2.1.1]己烷通过光化学 [2π + 2σ] 环加成反应由 gem-二氟二烯的氧化活化引发
将氟原子掺入三维富含 sp3 的支架中代表了生物等排进化活动中的一种有吸引力的策略,它赋予生物等排候选物改进的药代动力学特性。光或路易斯酸介导的双环[1.1.0]丁烷环加成反应为构建许多常规双环[n.1.1]支架(n = 1-5)提供了一种有效的方法,但仍然是合成相关3D氟化支架的重大挑战。在此,我们揭示了一种用于 gem-二氟二烯活化和随后的 [2π + 2σ] 环加成与双环 [1.1.0] 丁烷的光化学单电子氧化策略,以提供范围广泛的 gem-二氟双环 [2.1.1] 己烷支架,其中包含几个后转换手柄。实验和计算机理研究的结合表明,gem-difluorodiene 的共轭 π 系统在环化过程中促进其优先的单电子氧化和稳定各种自由基参与中间体方面发挥着重要的双重作用。
更新日期:2024-11-15
中文翻译:

gem-二氟双环[2.1.1]己烷通过光化学 [2π + 2σ] 环加成反应由 gem-二氟二烯的氧化活化引发
将氟原子掺入三维富含 sp3 的支架中代表了生物等排进化活动中的一种有吸引力的策略,它赋予生物等排候选物改进的药代动力学特性。光或路易斯酸介导的双环[1.1.0]丁烷环加成反应为构建许多常规双环[n.1.1]支架(n = 1-5)提供了一种有效的方法,但仍然是合成相关3D氟化支架的重大挑战。在此,我们揭示了一种用于 gem-二氟二烯活化和随后的 [2π + 2σ] 环加成与双环 [1.1.0] 丁烷的光化学单电子氧化策略,以提供范围广泛的 gem-二氟双环 [2.1.1] 己烷支架,其中包含几个后转换手柄。实验和计算机理研究的结合表明,gem-difluorodiene 的共轭 π 系统在环化过程中促进其优先的单电子氧化和稳定各种自由基参与中间体方面发挥着重要的双重作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号