Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Preparation of MnO2 on the Catechol/Silane-Coated Polypropylene Nonwoven Fabrics for Effective Removal of Cationic Dyes
Langmuir ( IF 3.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.langmuir.4c03266 Jing Li, Hui Sun, Dewei Zhang, Xiaodong Yang, Zhuan Fu, Bin Yu
Langmuir ( IF 3.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.langmuir.4c03266 Jing Li, Hui Sun, Dewei Zhang, Xiaodong Yang, Zhuan Fu, Bin Yu
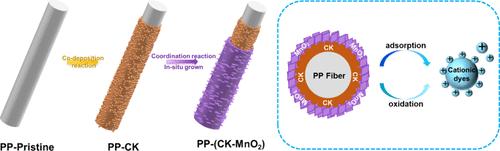
|
Previous studies have confirmed that MnOx removes heavy metal ions and organic pollutants from water with dual effects of adsorption and oxidation coupling, significantly improving the ability to remove impurities. Nanometal oxides have a highly reactive surface but tend to agglomerate during preparation and are challenging to recycle after use. A common method is to combine nano-MnO2 with Fe3O4 to prepare magnetic materials for easy recycling. Our previous research has confirmed that catechol (CA) and (3-aminopropyl) triethoxysilane (KH550) can be co-deposited on the surface of polypropylene nonwovens to form a stable CK coating under alkaline conditions. In addition, the coating has many active groups, including hydroxyl groups, amino groups, etc. This study further investigates the secondary reactivity of CK coatings. The coordination of catechol groups and metal ions was used to anchor manganese ions to the coating. Meanwhile, the hydroxyl and amino groups were used to reduce manganese ions to Mn4+ in situ to prepare PP-(CK-MnO2). We found that the sample had an excellent decolorization effect on cationic dyes but was limited to anionic dyes. The decolorization mechanism of cationic dyes was further discussed. The results showed that the decolorization of cationic dyes had a dual effect of adsorption and oxidative degradation. Under acidic conditions, its oxidation properties were enhanced. It can be used as a highly effective decolorizing agent for cationic dyes, and the decolorization behavior is consistent with the first-order kinetics. As the pH increases, its oxidation properties gradually decrease. Although the electrostatic adsorption effect was enhanced, the overall decolorization performance was significantly reduced. Recycling experiments have proved that it can maintain >90% removal rate after five cycles. This study also demonstrated that the CK coating has dopamine-like properties, which can coordinate with metal ions to prepare metal–organic hybrid materials.
中文翻译:

在邻苯二酚/硅烷涂层聚丙烯无纺布上原位制备 MnO2 以有效去除阳离子染料
以往的研究证实,MnOx 具有吸附和氧化耦合的双重作用,可以去除水中的重金属离子和有机污染物,显著提高去除杂质的能力。纳米金属氧化物具有高度反应性的表面,但在制备过程中容易结块,使用后难以回收。一种常见的方法是将纳米 MnO2 与 Fe3O4 结合,制备易于回收的磁性材料。我们之前的研究已经证实,邻苯二酚 (CA) 和(3-氨丙基)三乙氧基硅烷 (KH550) 可以在碱性条件下共沉积在聚丙烯非织造布的表面,形成稳定的 CK 涂层。此外,涂层中有许多活性基团,包括羟基、氨基等。本研究进一步研究了 CK 涂层的次级反应性。邻苯二酚基团和金属离子的配位用于将锰离子锚定到涂层上。同时,利用羟基和氨基原位将锰离子还原为 Mn4+,制备 PP-(CK-MnO2)。我们发现该样品对阳离子染料具有极好的脱色效果,但仅限于阴离子染料。进一步讨论了阳离子染料的脱色机理。结果表明,阳离子染料的脱色具有吸附和氧化降解的双重作用。在酸性条件下,其氧化性能增强。可用作阳离子染料的高效脱色剂,脱色行为与一级动力学一致。随着 pH 值的增加,其氧化性能逐渐降低。 虽然静电吸附效果增强,但整体脱色性能明显降低。回收实验证明,5次循环后仍能保持>90%的去除率。这项研究还表明,CK 涂层具有类似多巴胺的特性,可以与金属离子配位制备金属-有机杂化材料。
更新日期:2024-11-14
中文翻译:

在邻苯二酚/硅烷涂层聚丙烯无纺布上原位制备 MnO2 以有效去除阳离子染料
以往的研究证实,MnOx 具有吸附和氧化耦合的双重作用,可以去除水中的重金属离子和有机污染物,显著提高去除杂质的能力。纳米金属氧化物具有高度反应性的表面,但在制备过程中容易结块,使用后难以回收。一种常见的方法是将纳米 MnO2 与 Fe3O4 结合,制备易于回收的磁性材料。我们之前的研究已经证实,邻苯二酚 (CA) 和(3-氨丙基)三乙氧基硅烷 (KH550) 可以在碱性条件下共沉积在聚丙烯非织造布的表面,形成稳定的 CK 涂层。此外,涂层中有许多活性基团,包括羟基、氨基等。本研究进一步研究了 CK 涂层的次级反应性。邻苯二酚基团和金属离子的配位用于将锰离子锚定到涂层上。同时,利用羟基和氨基原位将锰离子还原为 Mn4+,制备 PP-(CK-MnO2)。我们发现该样品对阳离子染料具有极好的脱色效果,但仅限于阴离子染料。进一步讨论了阳离子染料的脱色机理。结果表明,阳离子染料的脱色具有吸附和氧化降解的双重作用。在酸性条件下,其氧化性能增强。可用作阳离子染料的高效脱色剂,脱色行为与一级动力学一致。随着 pH 值的增加,其氧化性能逐渐降低。 虽然静电吸附效果增强,但整体脱色性能明显降低。回收实验证明,5次循环后仍能保持>90%的去除率。这项研究还表明,CK 涂层具有类似多巴胺的特性,可以与金属离子配位制备金属-有机杂化材料。































 京公网安备 11010802027423号
京公网安备 11010802027423号