当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-Innocent Role of Sacrificial Anodes in Electrochemical Nickel-Catalyzed C(sp2)–C(sp3) Cross-Electrophile Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c10979 Luana Cardinale, Gregory L. Beutner, Christopher Y. Bemis, Daniel J. Weix, Shannon S. Stahl
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c10979 Luana Cardinale, Gregory L. Beutner, Christopher Y. Bemis, Daniel J. Weix, Shannon S. Stahl
|
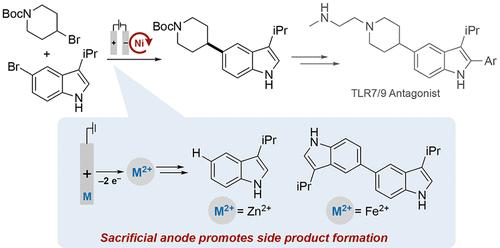
Sacrificial anodes composed of inexpensive metals such as Zn, Fe, and Mg are widely used to support electrochemical nickel-catalyzed cross-electrophile coupling (XEC) reactions, in addition to other reductive electrochemical transformations. Such anodes are appealing because they provide a stable counter-electrode potential and typically avoid interference with the reductive chemistry. The present study outlines the development of an electrochemical Ni-catalyzed XEC reaction that streamlines access to a key pharmaceutical intermediate. Metal ions derived from sacrificial anode oxidation, however, directly contribute to homocoupling and proto-dehalogenation side products that are commonly formed in chemical and electrochemical Ni-catalyzed XEC reactions. Use of a divided cell limits interference by the anode-derived metal ions and supports a high product yield with negligible side product formation, introducing a strategy to overcome one of the main limitations of Ni-catalyzed XEC.
中文翻译:

牺牲阳极在电化学镍催化的 C(sp2)–C(sp3) 交叉亲电偶联中的非无辜作用
由锌、铁和镁等廉价金属组成的牺牲阳极被广泛用于支持电化学镍催化的交叉亲电耦合 (XEC) 反应,以及其他还原性电化学转变。这种阳极很有吸引力,因为它们提供稳定的对电极电位,并且通常避免干扰还原化学。本研究概述了电化学 Ni 催化的 XEC 反应的开发,该反应简化了关键药物中间体的获取。然而,来自牺牲阳极氧化的金属离子直接导致同偶联和原始脱卤副产物,这些副产物通常在化学和电化学镍催化的 XEC 反应中形成。使用分流电池限制了阳极衍生金属离子的干扰,并支持高产品产量,副产物形成可以忽略不计,引入了一种克服镍催化 XEC 主要限制之一的策略。
更新日期:2024-11-15
中文翻译:

牺牲阳极在电化学镍催化的 C(sp2)–C(sp3) 交叉亲电偶联中的非无辜作用
由锌、铁和镁等廉价金属组成的牺牲阳极被广泛用于支持电化学镍催化的交叉亲电耦合 (XEC) 反应,以及其他还原性电化学转变。这种阳极很有吸引力,因为它们提供稳定的对电极电位,并且通常避免干扰还原化学。本研究概述了电化学 Ni 催化的 XEC 反应的开发,该反应简化了关键药物中间体的获取。然而,来自牺牲阳极氧化的金属离子直接导致同偶联和原始脱卤副产物,这些副产物通常在化学和电化学镍催化的 XEC 反应中形成。使用分流电池限制了阳极衍生金属离子的干扰,并支持高产品产量,副产物形成可以忽略不计,引入了一种克服镍催化 XEC 主要限制之一的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号