当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C–OH Bond Activation for Stereoselective Radical C-Glycosylation of Native Saccharides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c11857 Hao Xie, Sheng Wang, Xing-Zhong Shu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-11-15 , DOI: 10.1021/jacs.4c11857 Hao Xie, Sheng Wang, Xing-Zhong Shu
|
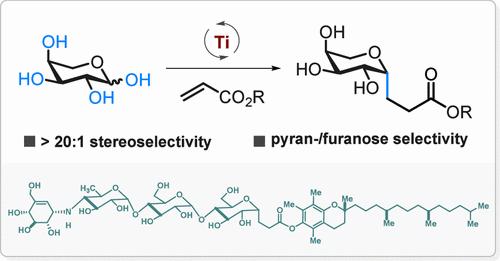
Radical C-glycosylation presents a flexible and efficient method for synthesizing C-glycosides. Existing methods always require multistep processes for generating anomeric radicals. In this study, we introduce a streamlined approach to produce anomeric radicals through direct C–OH bond homolysis of unmodified saccharides, eliminating the need for protection, deprotection, or activation steps. These anomeric radicals selectively couple with activated alkenes, yielding C-glycosylation products with high stereoselectivity (>20:1). This method is applicable to a variety of native monosaccharides, such as l-arabinose, d-arabinose, d-xylose, l-xylose, d-galactose, β-d-glucose, α-d-glucose, and l-ribose, as well as oligosaccharides including α-lactose, d-(+)-melibiose, and acarbose. We also extend this approach to C-glycosylation of amino acid and peptide derivatives, and demonstrate a streamlined synthesis of an anti-inflammatory agent.
中文翻译:

天然糖类立体选择性自由基 C-糖基化的 C-OH 键激活
自由基 C-糖基化提供了一种灵活高效的合成 C-糖苷的方法。现有方法总是需要多步骤过程来生成异头自由基。在这项研究中,我们介绍了一种简化的方法,通过未修饰糖类的直接 C-OH 键同解来产生异头自由基,无需保护、脱保护或激活步骤。这些异头自由基选择性地与活化的烯烃偶联,产生具有高立体选择性 (>20:1) 的 C-糖基化产物。该方法适用于多种天然单糖,如 l-阿拉伯糖、d-阿拉伯糖、d-木糖、l-木糖、d-半乳糖、β-d-葡萄糖、α-d-葡萄糖和 l-核糖,以及低聚糖,包括 α-乳糖、d-(+)-甲基二糖和阿卡波糖。我们还将这种方法扩展到氨基酸和肽衍生物的 C-糖基化,并展示了抗炎剂的简化合成。
更新日期:2024-11-15
中文翻译:

天然糖类立体选择性自由基 C-糖基化的 C-OH 键激活
自由基 C-糖基化提供了一种灵活高效的合成 C-糖苷的方法。现有方法总是需要多步骤过程来生成异头自由基。在这项研究中,我们介绍了一种简化的方法,通过未修饰糖类的直接 C-OH 键同解来产生异头自由基,无需保护、脱保护或激活步骤。这些异头自由基选择性地与活化的烯烃偶联,产生具有高立体选择性 (>20:1) 的 C-糖基化产物。该方法适用于多种天然单糖,如 l-阿拉伯糖、d-阿拉伯糖、d-木糖、l-木糖、d-半乳糖、β-d-葡萄糖、α-d-葡萄糖和 l-核糖,以及低聚糖,包括 α-乳糖、d-(+)-甲基二糖和阿卡波糖。我们还将这种方法扩展到氨基酸和肽衍生物的 C-糖基化,并展示了抗炎剂的简化合成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号