当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Antifungal Activity Evaluation of Novel L-Carvone-Based 1,3,4-Thiadiazole-amide Derivatives as a Potential Succinate Dehydrogenase Inhibitor
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.jafc.4c06916 Baoyu Li, Wengui Duan, Guishan Lin, Xinyan Liu, Yucheng Cui, Yin Man
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.jafc.4c06916 Baoyu Li, Wengui Duan, Guishan Lin, Xinyan Liu, Yucheng Cui, Yin Man
|
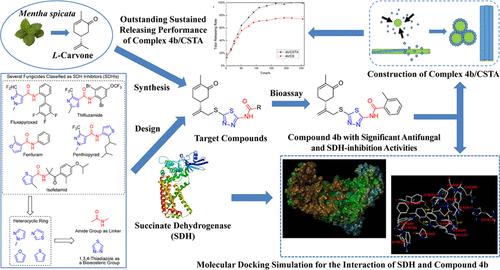
In an effort to explore new-type high-efficiency antifungal agents, 25 novel L-carvone-based 1,3,4-thiadiazole-amide derivatives were designed, synthesized, and structurally characterized by IR, 1H NMR, 13C NMR, and high-resolution mass spectrometry (HRMS) analyses. The antifungal activity of the target compounds was preliminarily assayed at a concentration of 50 μg/mL, and boscalid, a commercialized fungicide identified as a succinate dehydrogenase inhibitor (SDHI), was employed as the positive control. It was found that all of the target compounds showed moderate to potent antifungal activity against the tested fungi compared to boscalid. Surprisingly, compound 4b exhibited broad-spectrum and significant inhibition activity against the growth of eight phytopathogenic strains with inhibitory rates of 67–89%. Further, the results of the EC50 value test suggested that the EC50 values of compound 4b against Physalospora piricola and Colletotrichum orbiculare were 16.33 and 18.06 μg/mL, respectively, and both of them were better than those of boscalid (16.64 and >50). Therefore, compound 4b deserves further study as a lead compound for novel fungicides. In addition, the inhibitory activity of compound 4b against succinate dehydrogenase (SDH) was evaluated as well to prove that compound 4b (IC50 = 3.38 μM) displayed higher SDH-inhibition activity than boscalid (IC50 = 7.02 μM). The binding mode of compound 4b and SDH was simulated by molecular docking and found to be similar to that of boscalid. The structure–activity relationships (SARs) of the target compounds were analyzed by establishing a 3D-QSAR model. Besides, a 4b-loaded complex 4b/CSTA on a reported L-carvone-based nanochitosan carrier CSTA containing the 1,3,4-thiadiazole-amide group was constructed, and its sustained release performance was investigated in the EtOH–H2O system (1:9, v/v). The complex 4b/CSTA exhibited preferred sustained release performance, indicating its potential for developing environmentally friendly nanofungicides.
中文翻译:

基于 L-香芹酮的新型 1,3,4-噻二唑酰胺衍生物作为潜在琥珀酸脱氢酶抑制剂的合成和抗真菌活性评价
为了探索新型高效抗真菌剂,设计、合成了 25 种基于 L-香芹酮的 1,3,4-噻二唑酰胺衍生物,并通过 IR、1H NMR、13C NMR 和高分辨率质谱 (HRMS) 分析进行了结构表征。在 50 μg/mL 的浓度下初步测定目标化合物的抗真菌活性,采用经鉴定为琥珀酸脱氢酶抑制剂 (SDHI) 的商业化杀菌剂 boscalid 作为阳性对照。结果发现,与啶酰菌胺相比,所有目标化合物对测试的真菌都显示出中等至强效的抗真菌活性。令人惊讶的是,化合物 4b 对 8 种植物病原菌株的生长表现出广谱和显著的抑制活性,抑制率为 67-89%。此外,EC50 值检验结果表明,化合物 4b 对 Physalospora piricola 和 Colletotrichum orbiculare 的 EC50 值分别为 16.33 和 18.06 μg/mL,均优于啶酰菌胺 (16.64 和 >50)。因此,化合物 4b 作为新型杀菌剂的先导化合物值得进一步研究。此外,还评估了化合物 4b 对琥珀酸脱氢酶 (SDH) 的抑制活性,以证明化合物 4b (IC50 = 3.38 μM) 显示出比啶酰菌胺 (IC50 = 7.02 μM) 更高的 SDH 抑制活性。通过分子对接模拟化合物 4b 和 SDH 的结合模式,发现与啶酰菌胺相似。通过建立 3D-QSAR 模型分析目标化合物的构效关系 (SARs)。 此外,在含有 1,3,4-噻二唑酰胺基团的已报道的基于 L-香芹酮的纳米壳聚糖载体 CSTA 上构建了 4b 负载的复合物 4b/CSTA,并在 EtOH–H2O 系统中研究了其缓释性能 (1:9, v/v)。复合物 4b/CSTA 表现出优异的缓释性能,表明其开发环保纳米杀菌剂的潜力。
更新日期:2024-11-14
中文翻译:

基于 L-香芹酮的新型 1,3,4-噻二唑酰胺衍生物作为潜在琥珀酸脱氢酶抑制剂的合成和抗真菌活性评价
为了探索新型高效抗真菌剂,设计、合成了 25 种基于 L-香芹酮的 1,3,4-噻二唑酰胺衍生物,并通过 IR、1H NMR、13C NMR 和高分辨率质谱 (HRMS) 分析进行了结构表征。在 50 μg/mL 的浓度下初步测定目标化合物的抗真菌活性,采用经鉴定为琥珀酸脱氢酶抑制剂 (SDHI) 的商业化杀菌剂 boscalid 作为阳性对照。结果发现,与啶酰菌胺相比,所有目标化合物对测试的真菌都显示出中等至强效的抗真菌活性。令人惊讶的是,化合物 4b 对 8 种植物病原菌株的生长表现出广谱和显著的抑制活性,抑制率为 67-89%。此外,EC50 值检验结果表明,化合物 4b 对 Physalospora piricola 和 Colletotrichum orbiculare 的 EC50 值分别为 16.33 和 18.06 μg/mL,均优于啶酰菌胺 (16.64 和 >50)。因此,化合物 4b 作为新型杀菌剂的先导化合物值得进一步研究。此外,还评估了化合物 4b 对琥珀酸脱氢酶 (SDH) 的抑制活性,以证明化合物 4b (IC50 = 3.38 μM) 显示出比啶酰菌胺 (IC50 = 7.02 μM) 更高的 SDH 抑制活性。通过分子对接模拟化合物 4b 和 SDH 的结合模式,发现与啶酰菌胺相似。通过建立 3D-QSAR 模型分析目标化合物的构效关系 (SARs)。 此外,在含有 1,3,4-噻二唑酰胺基团的已报道的基于 L-香芹酮的纳米壳聚糖载体 CSTA 上构建了 4b 负载的复合物 4b/CSTA,并在 EtOH–H2O 系统中研究了其缓释性能 (1:9, v/v)。复合物 4b/CSTA 表现出优异的缓释性能,表明其开发环保纳米杀菌剂的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号