当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manganese-Catalyzed Asymmetric Hydrogenation for Atroposelective Dynamic Kinetic Resolution of Heterobiaryl Ketone N-Oxides
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c04979 Yin-Bo Wan, Xiang-Ping Hu
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-15 , DOI: 10.1021/acscatal.4c04979 Yin-Bo Wan, Xiang-Ping Hu
|
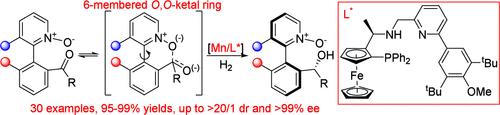
An atroposelective dynamic kinetic resolution of configurationally labile heterobiaryl ketone N-oxides via Mn-catalyzed asymmetric hydrogenation has been disclosed. By use of a structurally finely tuned chiral ferrocenyl P,N,N-ligand, the hydrogenation proceeds smoothly under mild conditions with simultaneous installation of central and axial chirality, giving a wide range of atropisomeric 1-arylisoquinoline and 2-arylpyridine N-oxides bearing a chiral alcohol structure with high diastereo- and enantioselectivities. The diastereomer of the hydrogenation product could be readily prepared in a stereospecific way with the complete inversion of the central chirality via Mitsunobu reaction. The value of this central- and axial-chiral heterobiaryl N-oxide scaffold is preliminarily demonstrated by its successful utility as a chiral catalyst in asymmetric allylation of benzaldehyde with allyltrichlorosilane.
中文翻译:

锰催化的不对称氢化反应用于异联芳基酮 N-氧化物的无选择性动态动力学分离
通过 Mn 催化的不对称氢化对构型不稳定的异联芳基酮 N-氧化物的无力选择性动态动力学分辨率已被披露。通过使用结构精细调整的手性二茂铁基 P,N,N-配体,氢化在温和的条件下顺利进行,同时安装中心和轴向手性,得到广泛的空斜异构体 1-芳基异喹啉和 2-芳基吡啶 N-氧化物,这些氧化物具有具有高非对映和对映选择性的手性醇结构。氢化产物的非对映异构体可以很容易地以立体特异性方式制备,并通过 Mitsunobu 反应完全反转中心手性。这种中心和轴向手性异联芳基 N-氧化物支架的价值初步证明在其作为苯甲醛与烯丙基三氯硅烷的不对称烯丙基化反应中的手性催化剂的成功实用。
更新日期:2024-11-15
中文翻译:

锰催化的不对称氢化反应用于异联芳基酮 N-氧化物的无选择性动态动力学分离
通过 Mn 催化的不对称氢化对构型不稳定的异联芳基酮 N-氧化物的无力选择性动态动力学分辨率已被披露。通过使用结构精细调整的手性二茂铁基 P,N,N-配体,氢化在温和的条件下顺利进行,同时安装中心和轴向手性,得到广泛的空斜异构体 1-芳基异喹啉和 2-芳基吡啶 N-氧化物,这些氧化物具有具有高非对映和对映选择性的手性醇结构。氢化产物的非对映异构体可以很容易地以立体特异性方式制备,并通过 Mitsunobu 反应完全反转中心手性。这种中心和轴向手性异联芳基 N-氧化物支架的价值初步证明在其作为苯甲醛与烯丙基三氯硅烷的不对称烯丙基化反应中的手性催化剂的成功实用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号