当前位置:
X-MOL 学术
›
Electrochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of ammoniacal thiosulfate solution composition on the gold dissolution rate: An electrochemical study
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-15 , DOI: 10.1016/j.electacta.2024.145359 José A. Tamayo, Carolina Ramírez-Sánchez, Jorge A. Calderón
Electrochimica Acta ( IF 5.5 ) Pub Date : 2024-11-15 , DOI: 10.1016/j.electacta.2024.145359 José A. Tamayo, Carolina Ramírez-Sánchez, Jorge A. Calderón
|
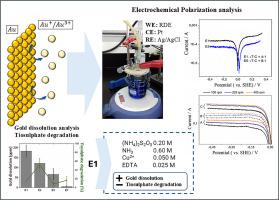
The electrochemical behavior of ammoniacal thiosulfate solutions (S2O32-- NH3-Cu2+ -EDTA) has been studied for varying electrolyte compositions in the gold electro-dissolution. A gold rotating disk electrode (RDE) was employed to measure anodic and cathodic polarization curves in alkaline thiosulfate solutions. Potentiodynamic polarization showed how thiosulfate, ammonia, and copper concentration influence the cathodic and anodic behavior of the electrolyte. Likewise, the influence of oxygen on the electrochemical behavior system was evidenced from the analysis of polarization curves, and using Koutecky – Levich equation (slope of 59.60 mV/decade), it was possible to determine the peroxide pathway for oxygen reduction reaction (ORR) with two-electron transference. Additionally, gold dissolution and thiosulfate degradation were evaluated using the gold foil leaching tests. It was found solutions with a 0.2 mol L-1 thiosulfate concentration favored the dissolution of gold and by maintaining an adequate ratio between thiosulfate/ammonium (1:3) or thiosulfate/copper (4:1) enable the attainment high gold dissolution and lower degradation of thiosulfate.
中文翻译:

硫代硫酸氨溶液成分对金溶解速率的影响:电化学研究
已经研究了硫代硫酸氨溶液 (S2O32-- NH3-Cu2+ -EDTA) 的电化学行为,用于金电溶解中不同的电解质组成。采用金旋转圆盘电极 (RDE) 测量碱性硫代硫酸盐溶液中的阳极和阴极极化曲线。动电位极化显示了硫代硫酸盐、氨和铜浓度如何影响电解质的阴极和阳极行为。同样,通过极化曲线的分析证明了氧对电化学行为系统的影响,并使用 Koutecky – Levich 方程(斜率为 59.60 mV/decade),可以确定具有双电子转移的氧还原反应 (ORR) 的过氧化物途径。此外,使用金箔浸出测试评估了金溶解和硫代硫酸盐降解。研究发现,浓度为 0.2 mol L-1 硫代硫酸盐的溶液有利于金的溶解,并且通过保持硫代硫酸盐/铵 (1:3) 或硫代硫酸盐/铜 (4:1) 之间的适当比例,可以实现高金溶解度和较低的硫代硫酸盐降解。
更新日期:2024-11-15
中文翻译:

硫代硫酸氨溶液成分对金溶解速率的影响:电化学研究
已经研究了硫代硫酸氨溶液 (S2O32-- NH3-Cu2+ -EDTA) 的电化学行为,用于金电溶解中不同的电解质组成。采用金旋转圆盘电极 (RDE) 测量碱性硫代硫酸盐溶液中的阳极和阴极极化曲线。动电位极化显示了硫代硫酸盐、氨和铜浓度如何影响电解质的阴极和阳极行为。同样,通过极化曲线的分析证明了氧对电化学行为系统的影响,并使用 Koutecky – Levich 方程(斜率为 59.60 mV/decade),可以确定具有双电子转移的氧还原反应 (ORR) 的过氧化物途径。此外,使用金箔浸出测试评估了金溶解和硫代硫酸盐降解。研究发现,浓度为 0.2 mol L-1 硫代硫酸盐的溶液有利于金的溶解,并且通过保持硫代硫酸盐/铵 (1:3) 或硫代硫酸盐/铜 (4:1) 之间的适当比例,可以实现高金溶解度和较低的硫代硫酸盐降解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号