当前位置:
X-MOL 学术
›
Aliment. Pharm. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Meta‐Analysis: Evaluating Placebo Rates Across Outcomes in Eosinophilic Oesophagitis Randomised Controlled Trials
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-11-15 , DOI: 10.1111/apt.18382 Angelica Rivas, Newaz Shubidito Ahmed, Yuhong Yuan, Anila Qasim, David B. O'Gorman, Brian G. Feagan, Vipul Jairath, Albert J. Bredenoord, Evan S. Dellon, Christopher Ma
Alimentary Pharmacology & Therapeutics ( IF 6.6 ) Pub Date : 2024-11-15 , DOI: 10.1111/apt.18382 Angelica Rivas, Newaz Shubidito Ahmed, Yuhong Yuan, Anila Qasim, David B. O'Gorman, Brian G. Feagan, Vipul Jairath, Albert J. Bredenoord, Evan S. Dellon, Christopher Ma
|
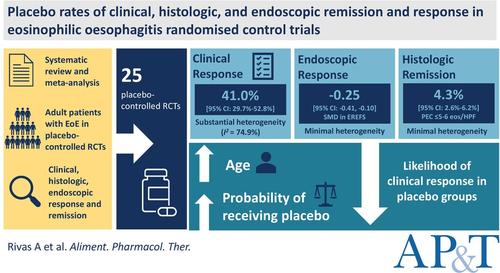
BackgroundHigh placebo responses have limited drug development in eosinophilic oesophagitis. The optimal configuration of trial outcomes is uncertain.AimsTo inform more efficient future trial designs, to characterise clinical, endoscopic and histologic placebo responses in eosinophilic oesophagitis randomised controlled trials (RCTs).MethodsWe updated a Cochrane systematic review and meta‐analysis, searching multiple databases to January 1, 2024, to identify placebo‐controlled RCTs evaluating medical therapies for patients with eosinophilic oesophagitis. The primary outcome was the pooled proportion of study‐defined clinical, endoscopic and histologic responders and remitters randomised to placebo, using an intention‐to‐treat approach and random‐effects model. Sources of heterogeneity were explored using meta‐regression.ResultsWe included 25 RCTs. The pooled proportion of clinical response was 41.0% [95% CI: 29.7%–52.8%] with substantial heterogeneity (I 2 = 74.9%). On meta‐regression, older age and a higher probability of being randomised to placebo reduced the likelihood of clinical response to placebo. The pooled proportion of histologic remission defined as a peak eosinophil count [PEC] ≤ 6 eosinophils per high power field [HPF] or ≤ 1 eosinophil/HPF was 4.3% [95% CI: 2.6%–6.2%] (I 2 = 23.6%) and 1.3% [95% CI: 0.5%–2.5%] (I 2 = 0%), respectively. The standardised mean difference in the Eosinophilic Oesophagitis Endoscopic Reference Score to placebo was −0.25 [95% CI: −0.41, −0.10].ConclusionsOver 40% of patients in eosinophilic oesophagitis trials respond clinically to placebo, and this is associated with trial design factors such as randomisation ratio and trial population. Objective endoscopic and histologic measures are associated with very low placebo responses.
中文翻译:

Meta 分析: 评估嗜酸性粒细胞性食管炎随机对照试验中不同结局的安慰剂率
背景高安慰剂反应限制了嗜酸性粒细胞性食管炎的药物开发。试验结果的最佳配置尚不确定。目的为未来更有效的试验设计提供信息,以描述嗜酸性粒细胞性食管炎随机对照试验 (RCT) 中的临床、内窥镜和组织学安慰剂反应。方法我们更新了 Cochrane 系统评价和荟萃分析,检索了多个数据库至 2024 年 1 月 1 日,以确定评估嗜酸性粒细胞性食管炎患者药物治疗的安慰剂对照 RCT。主要结局是使用意向性治疗方法和随机效应模型,将研究定义的临床、内窥镜和组织学反应者和缓解者的合并比例随机分配到安慰剂组。使用 meta 回归探索异质性的来源。结果我们纳入了 25 项 RCT。临床反应的合并比例为 41.0% [95% CI: 29.7%–52.8%],存在很大异质性 (I2 = 74.9%)。在 meta 回归中,年龄较大和被随机分配到安慰剂组的可能性较高降低了对安慰剂的临床反应的可能性。定义为嗜酸性粒细胞峰值计数 [PEC] ≤每个高倍视野 [HPF] 或 1 个嗜酸性粒细胞/HPF ≤组织学缓解的合并比例分别为 4.3% [95% CI:2.6%–6.2%] (I2 = 23.6%) 和 1.3% [95% CI:0.5%–2.5%] (I2 = 0%)。嗜酸性粒细胞性食管炎内窥镜参考评分与安慰剂的标准化平均差为 -0.25 [95% CI: -0.41, -0.10]。结论嗜酸性粒细胞性食管炎试验中超过 40% 的患者对安慰剂有临床反应,这与随机化比和试验人群等试验设计因素有关。客观的内窥镜和组织学测量与非常低的安慰剂反应相关。
更新日期:2024-11-15
中文翻译:

Meta 分析: 评估嗜酸性粒细胞性食管炎随机对照试验中不同结局的安慰剂率
背景高安慰剂反应限制了嗜酸性粒细胞性食管炎的药物开发。试验结果的最佳配置尚不确定。目的为未来更有效的试验设计提供信息,以描述嗜酸性粒细胞性食管炎随机对照试验 (RCT) 中的临床、内窥镜和组织学安慰剂反应。方法我们更新了 Cochrane 系统评价和荟萃分析,检索了多个数据库至 2024 年 1 月 1 日,以确定评估嗜酸性粒细胞性食管炎患者药物治疗的安慰剂对照 RCT。主要结局是使用意向性治疗方法和随机效应模型,将研究定义的临床、内窥镜和组织学反应者和缓解者的合并比例随机分配到安慰剂组。使用 meta 回归探索异质性的来源。结果我们纳入了 25 项 RCT。临床反应的合并比例为 41.0% [95% CI: 29.7%–52.8%],存在很大异质性 (I2 = 74.9%)。在 meta 回归中,年龄较大和被随机分配到安慰剂组的可能性较高降低了对安慰剂的临床反应的可能性。定义为嗜酸性粒细胞峰值计数 [PEC] ≤每个高倍视野 [HPF] 或 1 个嗜酸性粒细胞/HPF ≤组织学缓解的合并比例分别为 4.3% [95% CI:2.6%–6.2%] (I2 = 23.6%) 和 1.3% [95% CI:0.5%–2.5%] (I2 = 0%)。嗜酸性粒细胞性食管炎内窥镜参考评分与安慰剂的标准化平均差为 -0.25 [95% CI: -0.41, -0.10]。结论嗜酸性粒细胞性食管炎试验中超过 40% 的患者对安慰剂有临床反应,这与随机化比和试验人群等试验设计因素有关。客观的内窥镜和组织学测量与非常低的安慰剂反应相关。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号