当前位置:
X-MOL 学术
›
Cell Metab.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Altered sphingolipid biosynthetic flux and lipoprotein trafficking contribute to trans-fat-induced atherosclerosis
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.cmet.2024.10.016 Jivani M. Gengatharan, Michal K. Handzlik, Zoya Y. Chih, Maureen L. Ruchhoeft, Patrick Secrest, Ethan L. Ashley, Courtney R. Green, Martina Wallace, Philip L.S.M. Gordts, Christian M. Metallo
Cell Metabolism ( IF 27.7 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.cmet.2024.10.016 Jivani M. Gengatharan, Michal K. Handzlik, Zoya Y. Chih, Maureen L. Ruchhoeft, Patrick Secrest, Ethan L. Ashley, Courtney R. Green, Martina Wallace, Philip L.S.M. Gordts, Christian M. Metallo
|
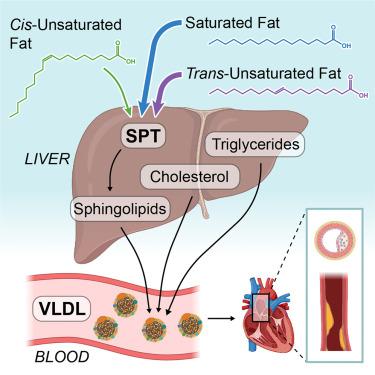
Dietary fat drives the pathogenesis of atherosclerotic cardiovascular disease (ASCVD), particularly through circulating cholesterol and triglyceride-rich lipoprotein remnants. Industrially produced trans-unsaturated fatty acids (TFAs) incorporated into food supplies significantly promote ASCVD. However, the molecular trafficking of TFAs responsible for this association is not well understood. Here, we demonstrate that TFAs are preferentially incorporated into sphingolipids by serine palmitoyltransferase (SPT) and secreted from cells in vitro. Administering high-fat diets (HFDs) enriched in TFAs to Ldlr−/− mice accelerated hepatic very-low-density lipoprotein (VLDL) and sphingolipid secretion into circulation to promote atherogenesis compared with a cis-unsaturated fatty acid (CFA)-enriched HFD. SPT inhibition mitigated these phenotypes and reduced circulating atherogenic VLDL enriched in TFA-derived polyunsaturated sphingomyelin. Transcriptional analysis of human liver revealed distinct regulation of SPTLC2 versus SPTLC3 subunit expression, consistent with human genetic correlations in ASCVD, further establishing sphingolipid metabolism as a critical node mediating the progression of ASCVD in response to specific dietary fats.
中文翻译:

改变的鞘脂生物合成通量和脂蛋白运输导致反式脂肪诱导的动脉粥样硬化
膳食脂肪驱动动脉粥样硬化性心血管疾病 (ASCVD) 的发病机制,特别是通过循环胆固醇和富含甘油三酯的脂蛋白残余物。食品供应中掺入工业生产的反式不饱和脂肪酸 (TFA) 可显著促进 ASCVD。然而,导致这种关联的 TFA 的分子运输尚不清楚。在这里,我们证明 TFA 优先通过丝氨酸棕榈酰转移酶 (SPT) 掺入鞘脂中,并在体外从细胞 中分泌。与富含顺式不饱和脂肪酸 (CFA) 的 HFD 相比,将富含 TFA 的高脂饮食 (HFD) 给予 Ldlr - / - 小鼠可加速肝脏极低密度脂蛋白 (VLDL) 和鞘脂分泌到循环中,以促进动脉粥样硬化生成。SPT 抑制减轻了这些表型,并减少了富含 TFA 衍生的多不饱和鞘磷脂的循环致动脉粥样硬化 VLDL。人肝脏的转录分析揭示了 SPTLC2 与 SPTLC3 亚基表达的不同调节,这与 ASCVD 中的人类遗传相关性一致,进一步确立了鞘脂代谢是介导 ASCVD 响应特定膳食脂肪进展的关键节点。
更新日期:2024-11-14
中文翻译:

改变的鞘脂生物合成通量和脂蛋白运输导致反式脂肪诱导的动脉粥样硬化
膳食脂肪驱动动脉粥样硬化性心血管疾病 (ASCVD) 的发病机制,特别是通过循环胆固醇和富含甘油三酯的脂蛋白残余物。食品供应中掺入工业生产的反式不饱和脂肪酸 (TFA) 可显著促进 ASCVD。然而,导致这种关联的 TFA 的分子运输尚不清楚。在这里,我们证明 TFA 优先通过丝氨酸棕榈酰转移酶 (SPT) 掺入鞘脂中,并在体外从细胞 中分泌。与富含顺式不饱和脂肪酸 (CFA) 的 HFD 相比,将富含 TFA 的高脂饮食 (HFD) 给予 Ldlr - / - 小鼠可加速肝脏极低密度脂蛋白 (VLDL) 和鞘脂分泌到循环中,以促进动脉粥样硬化生成。SPT 抑制减轻了这些表型,并减少了富含 TFA 衍生的多不饱和鞘磷脂的循环致动脉粥样硬化 VLDL。人肝脏的转录分析揭示了 SPTLC2 与 SPTLC3 亚基表达的不同调节,这与 ASCVD 中的人类遗传相关性一致,进一步确立了鞘脂代谢是介导 ASCVD 响应特定膳食脂肪进展的关键节点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号