当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pericancerous cross-presentation to cytotoxic T lymphocytes impairs immunotherapeutic efficacy in hepatocellular carcinoma
Cancer Cell ( IF 48.8 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.ccell.2024.10.012 Chun-Xiang Huang, Xiang-Ming Lao, Xu-Yan Wang, Yi-Zheng Ren, Yi-Tong Lu, Wei Shi, Ying-Zhe Wang, Cai-Yuan Wu, Li Xu, Min-Shan Chen, Qiang Gao, Lianxin Liu, Yuan Wei, Dong-Ming Kuang
Cancer Cell ( IF 48.8 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.ccell.2024.10.012 Chun-Xiang Huang, Xiang-Ming Lao, Xu-Yan Wang, Yi-Zheng Ren, Yi-Tong Lu, Wei Shi, Ying-Zhe Wang, Cai-Yuan Wu, Li Xu, Min-Shan Chen, Qiang Gao, Lianxin Liu, Yuan Wei, Dong-Ming Kuang
|
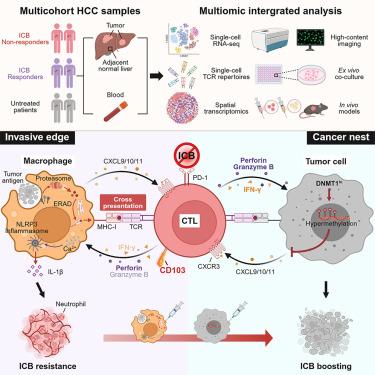
Hyperprogressive disease can occur in cancer patients receiving immune checkpoint blockade (ICB) therapy, but whether and how reactive cytotoxic T lymphocytes (CTLs) exert protumorigenic effects in this context remain elusive. Herein, our study reveals that pericancerous macrophages cross-present antigens to CD103+ CTLs in hepatocellular carcinoma (HCC) via the endoplasmic reticulum (ER)-associated degradation machinery-mediated cytosolic pathway. This process leads to the retention of CD103+ CTLs in the pericancerous area, whereby they activate NLRP3 inflammasome in macrophages, promoting hepatoma progression and resistance to immunotherapy. Our single-cell RNA sequencing (scRNA-seq) and spatial transcriptomics analysis of HCC patients shows that despite their tissue-resident effector phenotype, the aggregation of CD103+ CTLs predicts unfavorable clinical outcomes for HCC patients receiving multiple types of treatment. Correspondingly, therapeutic strategies that redistribute CD103+ CTLs can disrupt this pathogenic interplay with macrophages, enhancing the efficacy of ICB treatment against HCC.
中文翻译:

细胞毒性 T 淋巴细胞的癌周交叉呈递损害了肝细胞癌的免疫治疗效果
接受免疫检查点阻断 (ICB) 治疗的癌症患者可发生疾病进行性高度进展,但在这种情况下,反应性细胞毒性 T 淋巴细胞 (CTL) 是否以及如何发挥促肿瘤作用仍难以捉摸。在此,我们的研究表明,癌周巨噬细胞通过内质网 (ER) 相关降解机制介导的胞质途径将抗原交叉呈递给肝细胞癌 (HCC) 中的 CD103 + CTL。这个过程导致 CD103 + CTL 保留在癌周区域,从而激活巨噬细胞中的 NLRP3 炎性小体,促进肝癌进展和对免疫治疗的耐药性。我们对 HCC 患者的单细胞 RNA 测序 (scRNA-seq) 和空间转录组学分析表明,尽管他们的组织驻留效应子表型,但 CD103+ CTLs 的聚集预示着接受多种治疗的 HCC 患者的不良临床结果。相应地,重新分配 CD103+ CTL 的治疗策略可以破坏这种与巨噬细胞的致病性相互作用,从而增强 ICB 治疗对 HCC 的疗效。
更新日期:2024-11-14
中文翻译:

细胞毒性 T 淋巴细胞的癌周交叉呈递损害了肝细胞癌的免疫治疗效果
接受免疫检查点阻断 (ICB) 治疗的癌症患者可发生疾病进行性高度进展,但在这种情况下,反应性细胞毒性 T 淋巴细胞 (CTL) 是否以及如何发挥促肿瘤作用仍难以捉摸。在此,我们的研究表明,癌周巨噬细胞通过内质网 (ER) 相关降解机制介导的胞质途径将抗原交叉呈递给肝细胞癌 (HCC) 中的 CD103 + CTL。这个过程导致 CD103 + CTL 保留在癌周区域,从而激活巨噬细胞中的 NLRP3 炎性小体,促进肝癌进展和对免疫治疗的耐药性。我们对 HCC 患者的单细胞 RNA 测序 (scRNA-seq) 和空间转录组学分析表明,尽管他们的组织驻留效应子表型,但 CD103+ CTLs 的聚集预示着接受多种治疗的 HCC 患者的不良临床结果。相应地,重新分配 CD103+ CTL 的治疗策略可以破坏这种与巨噬细胞的致病性相互作用,从而增强 ICB 治疗对 HCC 的疗效。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号