当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigations on Cp*CoIII-Catalyzed Quinoline Transfer Hydrogenation with Formic Acid
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acscatal.4c05271 Nidhi Garg, Pardeep Dahiya, Sonia Mallet-Ladeira, Rinaldo Poli, Basker Sundararaju
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acscatal.4c05271 Nidhi Garg, Pardeep Dahiya, Sonia Mallet-Ladeira, Rinaldo Poli, Basker Sundararaju
|
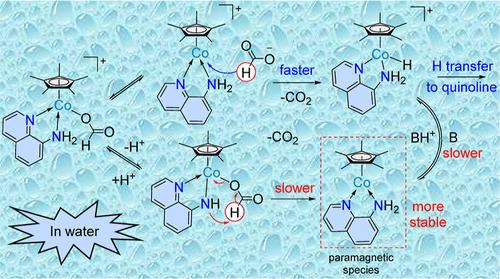
The mechanism of the quinoline transfer hydrogenation (TH) by aqueous HCOOH under the action of [Cp*Co(quinNH2)I]+ (A*; quinNH2 = 8-aminoquinoline) has been investigated by a combination of experiments and density functional theory (DFT) calculations. Variable-temperature (−40 to 20 °C) 1H NMR in the absence of quinoline substrate shows rapid equilibration between A* and the formate complex [Cp*Co(quinNH2)(O2CH)]+ (B*) upon the addition of HCOOH/NEt3 in MeOH, yielding ΔH° = 1.49 ± 0.03 kcal mol–1 and ΔS° = 1.92 ± 0.06 cal mol–1 K–1. This equilibrium mixture slowly converts by decarboxylation and deprotonation to paramagnetic (S = 1) [Cp*Cp(quinNH2)] (C*), indirectly identified by derivatization to [Cp*Co(CNtBu)2] and further I2 oxidation to [Cp*Co(CNtBu)2I](I3). The rate law of the [Cp*Co(quinNH2)I]+-catalyzed 8-methylquinoline (8MQ) TH with HCOOH in D2O at 80 °C has order one for substrate and catalyst and order zero for HCOOH, with a rate constant k = (1.52 ± 0.05) × 10–2 s–1 mol–1 L. The quinoline (Q) TH with HCOOH in D2O at 80 °C (k = (2.04 ± 0.05) × 10–2 s–1 mol–1 L) selectively yields tetrahydroquinoline doubly D-labeled at the C3 position ([3,3-D2]-THQ). Under the same conditions, DCOOD in D2O yields [2,3,3,4-D4]-THQ with k = (6.6 ± 0.6) × 10–3 s–1 mol–1 L (KIE = kH/kD = 3.1 ± 0.5), while DCOOD in H2O yields [2,4-D2]-THQ. DFT calculations of the Cp model system point to a catalytic cycle with both diamagnetic and paramagnetic intermediates. A key aspect is that the transfer of the formate H atom as a hydride to the metal center, converting [CpCo(quinNH2)(O2CH)]+ (B) to [CpCo(quinNH2)H]+ (D), is faster than its transfer as a proton to yield [CpCp(quinNH2)] (C). This is at variance with the closely related complex with the 8-hydroxyquinoline ligand (ACS Catal. 2021, 11, 11906–11920), underlining the decisive roles of ligand and reaction medium in the selection of the dehydrogenation pathway.
中文翻译:

Cp*CoIII 催化的喹啉转移加氢与甲酸的机理研究
通过实验和密度泛函理论 (DFT) 计算的结合,研究了在 [Cp*Co(quinNH2)I]+(A*;quinNH2 = 8-氨基喹啉)作用下,HCOOH 水溶液转移氢化 (TH) 的机理。在没有喹啉底物的情况下,变温(-40 至 20 °C)1H NMR 显示,在 MeOH 中加入 HCOOH/NEt3 后,A* 和甲酸盐络合物 [Cp*Co(quinNH2)(O2CH)]+ (B*) 之间快速平衡,产生 ΔH° = 1.49 ± 0.03 kcal mol–1 和 ΔS° = 1.92 ± 0.06 cal mol–1 K–1.这种平衡混合物通过脱羧和去质子化缓慢转化为顺磁性 (S = 1) [Cp*Cp(quinNH2)] (C*),通过衍生化为 [Cp*Co(CNtBu)2] 和进一步 I2 氧化为 [Cp*Co(CNtBu)2I](I3) 来间接鉴定。在 80 °C 下,在 D2O 中,含 HCOOH 的 [Cp*Co(quinNH2)I]+-催化的 8-甲基喹啉 (8MQ) TH 的速率定律对于底物和催化剂为 1 阶,对于 HCOOH 为 0 阶,速率常数 k = (1.52 ± 0.05) × 10–2 s–1 mol–1 L。在 80 °C 的 D2O 中含 HCOOH 的喹啉 (±Q) TH × 10–2 s–1 mol–1 L) 选择性地产生在 C3 位点 ([3,3-D2]-THQ) 处双 D 标记的四氢喹啉。 在相同条件下,D2O 中的 DCOOD 产生 [2,3,3,4-D4]-THQ,k = (6.6 ± 0.6) × 10-3 s–1 mol–1 L (KIE = kH/kD = 3.1 ± 0.5),而 H2O 中的 DCOOD 产生 [2,4-D2]-THQ。Cp 模型系统的 DFT 计算表明,抗磁和顺磁中间体都存在催化循环。一个关键方面是甲酸盐 H 原子作为氢化物转移到金属中心,将 [CpCo(quinNH2)(O2CH)]+ (B) 转化为 [CpCo(quinNH2)H]+ (D),比它作为质子转移产生 [CpCp(quinNH2)] (C) 更快。这与与 8-羟基喹啉配体 (ACS Catal.2021,11, 11906–11920),强调了配体和反应介质在脱氢途径选择中的决定性作用。
更新日期:2024-11-14
中文翻译:

Cp*CoIII 催化的喹啉转移加氢与甲酸的机理研究
通过实验和密度泛函理论 (DFT) 计算的结合,研究了在 [Cp*Co(quinNH2)I]+(A*;quinNH2 = 8-氨基喹啉)作用下,HCOOH 水溶液转移氢化 (TH) 的机理。在没有喹啉底物的情况下,变温(-40 至 20 °C)1H NMR 显示,在 MeOH 中加入 HCOOH/NEt3 后,A* 和甲酸盐络合物 [Cp*Co(quinNH2)(O2CH)]+ (B*) 之间快速平衡,产生 ΔH° = 1.49 ± 0.03 kcal mol–1 和 ΔS° = 1.92 ± 0.06 cal mol–1 K–1.这种平衡混合物通过脱羧和去质子化缓慢转化为顺磁性 (S = 1) [Cp*Cp(quinNH2)] (C*),通过衍生化为 [Cp*Co(CNtBu)2] 和进一步 I2 氧化为 [Cp*Co(CNtBu)2I](I3) 来间接鉴定。在 80 °C 下,在 D2O 中,含 HCOOH 的 [Cp*Co(quinNH2)I]+-催化的 8-甲基喹啉 (8MQ) TH 的速率定律对于底物和催化剂为 1 阶,对于 HCOOH 为 0 阶,速率常数 k = (1.52 ± 0.05) × 10–2 s–1 mol–1 L。在 80 °C 的 D2O 中含 HCOOH 的喹啉 (±Q) TH × 10–2 s–1 mol–1 L) 选择性地产生在 C3 位点 ([3,3-D2]-THQ) 处双 D 标记的四氢喹啉。 在相同条件下,D2O 中的 DCOOD 产生 [2,3,3,4-D4]-THQ,k = (6.6 ± 0.6) × 10-3 s–1 mol–1 L (KIE = kH/kD = 3.1 ± 0.5),而 H2O 中的 DCOOD 产生 [2,4-D2]-THQ。Cp 模型系统的 DFT 计算表明,抗磁和顺磁中间体都存在催化循环。一个关键方面是甲酸盐 H 原子作为氢化物转移到金属中心,将 [CpCo(quinNH2)(O2CH)]+ (B) 转化为 [CpCo(quinNH2)H]+ (D),比它作为质子转移产生 [CpCp(quinNH2)] (C) 更快。这与与 8-羟基喹啉配体 (ACS Catal.2021,11, 11906–11920),强调了配体和反应介质在脱氢途径选择中的决定性作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号