当前位置:
X-MOL 学术
›
Ann. N. Y. Acad. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The economics of translating a biosimilar from lab to market in India
Annals of the New York Academy of Sciences ( IF 4.1 ) Pub Date : 2024-11-13 , DOI: 10.1111/nyas.15252 Sonia Gandhi, Dhananjay Patankar, Smita Kashiramka, Anurag S. Rathore
Annals of the New York Academy of Sciences ( IF 4.1 ) Pub Date : 2024-11-13 , DOI: 10.1111/nyas.15252 Sonia Gandhi, Dhananjay Patankar, Smita Kashiramka, Anurag S. Rathore
|
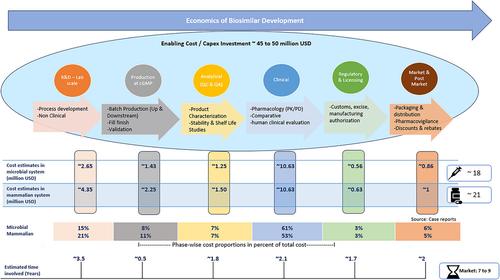
This study aims to establish a cost basis for biologics manufacturers and policymakers by quantifying the price and time required to bring a biosimilar from the lab to market. For efficient implementation of a cost-based policy, especially for life-saving medicines like biosimilars, it is imperative to establish a benchmark for the cost involved in biosimilar development. In this holistic and multiple-case study, stage-wise cost estimates of biosimilar development were obtained for microbial and mammalian systems. The investigation of six biopharmaceutical companies based in India concluded that biosimilar development through the microbial system costs ∼18 million USD and ∼21 million USD for the mammalian system. Additionally, 45–50 million USD is required as a one-time capital investment. Further, US/EU authorization can cost ∼25 million USD per product. Clinical studies are the most expensive and account for 60%–70% of total development cost. The presented information can serve as a basis for implementing cost-based pricing in countries like India and reimbursement policies for biosimilars under Medicare Part B in the United States.
中文翻译:

在印度将生物仿制药从实验室转化到市场的经济性
本研究旨在通过量化生物仿制药从实验室推向市场所需的价格和时间,为生物制剂制造商和政策制定者建立成本基础。为了有效实施基于成本的政策,特别是对于生物仿制药等拯救生命的药物,必须为生物仿制药开发所涉及的成本建立基准。在这项整体和多案例研究中,获得了微生物和哺乳动物系统的生物仿制药开发的阶段成本估计。对印度六家生物制药公司的调查得出结论,通过微生物系统开发生物仿制药的成本约为 1800 万美元,哺乳动物系统的成本约为 2100 万美元。此外,一次性资本投资需要 45-5000 万美元。此外,美国/欧盟授权每件产品的成本约为 2500 万美元。临床研究是最昂贵的,占总开发成本的 60%-70%。提供的信息可以作为在印度等国家实施基于成本的定价以及在美国根据 Medicare B 部分实施生物仿制药报销政策的基础。
更新日期:2024-11-13
中文翻译:

在印度将生物仿制药从实验室转化到市场的经济性
本研究旨在通过量化生物仿制药从实验室推向市场所需的价格和时间,为生物制剂制造商和政策制定者建立成本基础。为了有效实施基于成本的政策,特别是对于生物仿制药等拯救生命的药物,必须为生物仿制药开发所涉及的成本建立基准。在这项整体和多案例研究中,获得了微生物和哺乳动物系统的生物仿制药开发的阶段成本估计。对印度六家生物制药公司的调查得出结论,通过微生物系统开发生物仿制药的成本约为 1800 万美元,哺乳动物系统的成本约为 2100 万美元。此外,一次性资本投资需要 45-5000 万美元。此外,美国/欧盟授权每件产品的成本约为 2500 万美元。临床研究是最昂贵的,占总开发成本的 60%-70%。提供的信息可以作为在印度等国家实施基于成本的定价以及在美国根据 Medicare B 部分实施生物仿制药报销政策的基础。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号