当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogenic cholesterol rewires lipid metabolism in hepatocellular carcinoma via the CSNK2A1-IGF2R Ser2484 axis
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.jare.2024.11.021
Ren-yi Su, Chen-hao Xu, Hai-jun Guo, Li-jun Meng, Jian-yong Zhuo, Nan Xu, Hui-gang Li, Chi-yu He, Xuan-yu Zhang, Zheng-xin Lian, Shuai Wang, Chenhao Cao, Ruhong Zhou, Di Lu, Shu-sen Zheng, Xu-yong Wei, Xiao Xu
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.jare.2024.11.021
Ren-yi Su, Chen-hao Xu, Hai-jun Guo, Li-jun Meng, Jian-yong Zhuo, Nan Xu, Hui-gang Li, Chi-yu He, Xuan-yu Zhang, Zheng-xin Lian, Shuai Wang, Chenhao Cao, Ruhong Zhou, Di Lu, Shu-sen Zheng, Xu-yong Wei, Xiao Xu
|
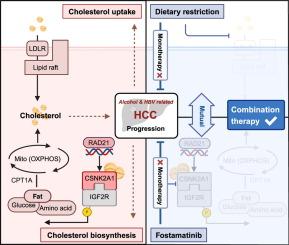
Alcohol consumption and hepatitis B virus (HBV) infection are common risk factors for hepatocellular carcinoma (HCC). However, few studies have focused on elucidating the mechanisms of HCC with combined alcohol and HBV etiology.
中文翻译:

致癌胆固醇通过 CSNK2A1-IGF2R Ser2484 轴重新连接肝细胞癌中的脂质代谢
饮酒和乙型肝炎病毒 (HBV) 感染是肝细胞癌 (HCC) 的常见危险因素。然而,很少有研究专注于阐明 HCC 与酒精和 HBV 联合病因的机制。
更新日期:2024-11-14
中文翻译:

致癌胆固醇通过 CSNK2A1-IGF2R Ser2484 轴重新连接肝细胞癌中的脂质代谢
饮酒和乙型肝炎病毒 (HBV) 感染是肝细胞癌 (HCC) 的常见危险因素。然而,很少有研究专注于阐明 HCC 与酒精和 HBV 联合病因的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号