当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deficiency of myeloid NPC1 exacerbates liver injury and fibrosis by impairing macrophage efferocytosis
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.jare.2024.11.020 Dongwei Guan, Pengju Huang, Xinlei Liu, Qing Li, Xiaoxun Zhang, Nan Liu, Yong Wang, Ying Wan, Jin Chai, Shiying Cai, Rui Chen, Zhijia Ye
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2024-11-14 , DOI: 10.1016/j.jare.2024.11.020 Dongwei Guan, Pengju Huang, Xinlei Liu, Qing Li, Xiaoxun Zhang, Nan Liu, Yong Wang, Ying Wan, Jin Chai, Shiying Cai, Rui Chen, Zhijia Ye
|
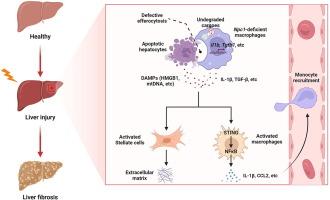
Niemann-Pick C1 (NPC1), a lysosomal cholesterol transport protein, is required for efficient efferocytosis. Patients with Npc1 mutation are frequently accompanied with hepatic symptoms, including hepatomegaly, elevated liver transaminases, or even acute liver failure, but the pathogenic mechanism remains unknown.
中文翻译:

髓系 NPC1 缺乏通过损害巨噬细胞胞吞作用加剧肝损伤和纤维化
Niemann-Pick C1 (NPC1) 是一种溶酶体胆固醇转运蛋白,是高效泡腾作用所必需的。Npc1 突变患者常伴有肝脏症状,包括肝肿大、肝转氨酶升高,甚至急性肝功能衰竭,但致病机制尚不清楚。
更新日期:2024-11-14
中文翻译:

髓系 NPC1 缺乏通过损害巨噬细胞胞吞作用加剧肝损伤和纤维化
Niemann-Pick C1 (NPC1) 是一种溶酶体胆固醇转运蛋白,是高效泡腾作用所必需的。Npc1 突变患者常伴有肝脏症状,包括肝肿大、肝转氨酶升高,甚至急性肝功能衰竭,但致病机制尚不清楚。






























 京公网安备 11010802027423号
京公网安备 11010802027423号