当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development and Biological Evaluation of New Diphenyl Ether Formylhydrazide Compounds as Potent Inhibitors of Succinate Dehydrogenase
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.jafc.4c07019 Bo He, Wang Chen, Lixiang Fu, Mengxu Hu, Zhenxi Xiong, Xianghui Luo, Yanhao Hu, Yalin Mu, Xu He, Wei Yan, Yonghao Ye
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.jafc.4c07019 Bo He, Wang Chen, Lixiang Fu, Mengxu Hu, Zhenxi Xiong, Xianghui Luo, Yanhao Hu, Yalin Mu, Xu He, Wei Yan, Yonghao Ye
|
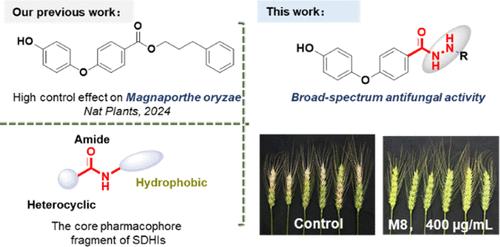
Succinate dehydrogenase (SDH), also recognized as succinate ubiquinone oxidoreductase (SQR), is considered one of the most promising targets for fungicide development, garnering significant international interest. We have focused on the development of highly effective, broad-spectrum-targeted SDH inhibitors. Using an active scaffold combining strategy, we designed and synthesized a series of novel diphenyl ether formylhydrazine derivatives, and most compounds have demonstrated broad-spectrum antifungal activity. Notably, compound M8 exhibited antifungal activity of more than 93% against four tested pathogen types at a concentration of 10 μg/mL, with an EC50 value below 0.3 μg/mL for each pathogen, outperforming boscalid. Additionally, compound M8 exhibited a control efficacy of 83% against Sclerotinia sclerotiorum on rapeseed leaves at a concentration of 200 μg/mL and demonstrated an 87% efficacy in controlling Fusarium graminearum on wheat ears when applied at 400 μg/mL. Structure–activity relationship research suggested that para-substituted benzene rings are more effective, offering stronger and more extensive antifungal potency. Further investigation, including enzyme inhibition assays, mycelial morphology observations, and molecular docking studies, suggests that the antifungal potency of M8 is due to the inhibition of its SDH activity. Therefore, our research positions compound M8 as a highly promising lead compound with broad-spectrum antifungal properties, potentially introducing a new class of fungicide.
中文翻译:

新型二苯醚甲酰肼化合物作为琥珀酸脱氢酶有效抑制剂的开发和生物学评价
琥珀酸脱氢酶 (SDH),也称为琥珀酸泛醌氧化还原酶 (SQR),被认为是杀菌剂开发最有前途的靶标之一,引起了国际社会的极大关注。我们专注于开发高效、广谱靶向的 SDH 抑制剂。使用活性支架组合策略,我们设计并合成了一系列新型二苯醚甲酰肼衍生物,大多数化合物已显示出广谱抗真菌活性。值得注意的是,化合物 M8 在浓度为 10 μg/mL 时对四种测试病原体类型的抗真菌活性超过 93%,每种病原体的 EC50 值低于 0.3 μg/mL,优于啶酰菌胺。此外,化合物 M8 在 200 μg/mL 的浓度下对油菜叶片上的菌核病菌表现出 83% 的控制效,当施用 400 μg/mL 时,对小麦穗上的禾谷镰刀菌的控制效能为 87%。构效关系研究表明,对位取代的苯环更有效,提供更强、更广泛的抗真菌效力。进一步的研究,包括酶抑制测定、菌丝体形态学观察和分子对接研究,表明 M8 的抗真菌效力是由于抑制其 SDH 活性。因此,我们的研究将化合物 M8 定位为一种非常有前途的先导化合物,具有广谱抗真菌特性,有可能引入一类新的杀菌剂。
更新日期:2024-11-14
中文翻译:

新型二苯醚甲酰肼化合物作为琥珀酸脱氢酶有效抑制剂的开发和生物学评价
琥珀酸脱氢酶 (SDH),也称为琥珀酸泛醌氧化还原酶 (SQR),被认为是杀菌剂开发最有前途的靶标之一,引起了国际社会的极大关注。我们专注于开发高效、广谱靶向的 SDH 抑制剂。使用活性支架组合策略,我们设计并合成了一系列新型二苯醚甲酰肼衍生物,大多数化合物已显示出广谱抗真菌活性。值得注意的是,化合物 M8 在浓度为 10 μg/mL 时对四种测试病原体类型的抗真菌活性超过 93%,每种病原体的 EC50 值低于 0.3 μg/mL,优于啶酰菌胺。此外,化合物 M8 在 200 μg/mL 的浓度下对油菜叶片上的菌核病菌表现出 83% 的控制效,当施用 400 μg/mL 时,对小麦穗上的禾谷镰刀菌的控制效能为 87%。构效关系研究表明,对位取代的苯环更有效,提供更强、更广泛的抗真菌效力。进一步的研究,包括酶抑制测定、菌丝体形态学观察和分子对接研究,表明 M8 的抗真菌效力是由于抑制其 SDH 活性。因此,我们的研究将化合物 M8 定位为一种非常有前途的先导化合物,具有广谱抗真菌特性,有可能引入一类新的杀菌剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号