当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism-Based Thermodynamic Analysis for One-Step and Two-Step Ethanol-to-1,3-Butadiene Conversion Processes
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.iecr.4c02681 Md Ziyaur Rahman, Abhishek R. Varma, Siddharth Gadkari, Atthasit Tawai, Malinee Sriariyanun, Vinod Kumar, Sunil K. Maity
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.iecr.4c02681 Md Ziyaur Rahman, Abhishek R. Varma, Siddharth Gadkari, Atthasit Tawai, Malinee Sriariyanun, Vinod Kumar, Sunil K. Maity
|
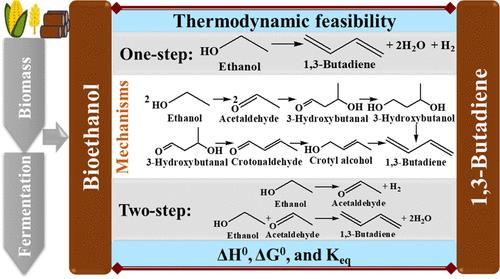
Renewable 1,3-butadiene (BD) is essential for sustainability of the synthetic rubber sector. This work presents a comprehensive thermodynamic analysis for one- and two-step ethanol-to-BD conversion processes. The two-step process comprises ethanol dehydrogenation, followed by the condensation of acetaldehyde with another ethanol molecule into BD. The process involves a complex reaction network with a wide range of byproducts depending on the nature of the catalysts and operating conditions, lacking unique consensus on the C–C bond-forming mechanism. This study elucidates the temperature regime for the spontaneity of the reactions proposed in various mechanisms and side reactions based on the standard Gibbs free energy change. The equilibrium conversion and product selectivity were further calculated under a wide temperature and pressure range. The overall reaction in the one-step process is thermodynamically spontaneous above 417 K, while the first and second steps of the two-step process are spontaneous above 550 and 285 K, respectively. Excepting Prins condensation, other mechanisms lack the spontaneity of all reaction steps. The equilibrium BD selectivity is favorable at elevated temperatures and low pressures. The addition of acetaldehyde in the two-step process has a favorable impact with higher BD selectivity, the maximum being at a 1:1 molar ratio of ethanol/acetaldehyde. This study elucidates thermodynamic insights into existing mechanisms and drives the evolution of a feasible mechanism. This effort will eventually help design novel catalysts and optimized processes for sustainable biobased BD production using ethanol derived from renewable feedstocks, aligning with the global commitment to greener and resource-friendly chemical manufacturing.
中文翻译:

基于机理的热力学分析,用于一步法和两步法乙醇制 1,3-丁二烯转化过程
可再生 1,3-丁二烯 (BD) 对于合成橡胶行业的可持续发展至关重要。这项工作为一步和两步乙醇到 BD 转化过程提出了全面的热力学分析。该两步过程包括乙醇脱氢,然后乙醛与另一个乙醇分子缩合成 BD。该工艺涉及一个复杂的反应网络,根据催化剂的性质和操作条件,会产生多种副产物,对 C-C 键形成机制缺乏独特的共识。本研究阐明了基于标准 Gibbs 自由能变化的各种机制和副反应中提出的反应自发性的温度状态。在较宽的温度和压力范围内进一步计算平衡转化率和产物选择性。一步法过程中的整体反应在 417 K 以上是热力学自发的,而两步法过程的第一步和第二步分别在 550 K 和 285 K 以上是自发的。除了 Prins 缩合外,其他机制缺乏所有反应步骤的自发性。平衡 BD 选择性在高温和低压下是有利的。在两步法中加入乙醛具有较高的 BD 选择性的有利影响,最大值为乙醇/乙醛的摩尔比为 1:1。本研究阐明了对现有机制的热力学见解,并推动了可行机制的演变。这项工作最终将有助于设计新型催化剂和优化工艺,使用来自可再生原料的乙醇进行可持续的生物基 BD 生产,这与全球对更绿色和资源友好型化学品制造的承诺保持一致。
更新日期:2024-11-14
中文翻译:

基于机理的热力学分析,用于一步法和两步法乙醇制 1,3-丁二烯转化过程
可再生 1,3-丁二烯 (BD) 对于合成橡胶行业的可持续发展至关重要。这项工作为一步和两步乙醇到 BD 转化过程提出了全面的热力学分析。该两步过程包括乙醇脱氢,然后乙醛与另一个乙醇分子缩合成 BD。该工艺涉及一个复杂的反应网络,根据催化剂的性质和操作条件,会产生多种副产物,对 C-C 键形成机制缺乏独特的共识。本研究阐明了基于标准 Gibbs 自由能变化的各种机制和副反应中提出的反应自发性的温度状态。在较宽的温度和压力范围内进一步计算平衡转化率和产物选择性。一步法过程中的整体反应在 417 K 以上是热力学自发的,而两步法过程的第一步和第二步分别在 550 K 和 285 K 以上是自发的。除了 Prins 缩合外,其他机制缺乏所有反应步骤的自发性。平衡 BD 选择性在高温和低压下是有利的。在两步法中加入乙醛具有较高的 BD 选择性的有利影响,最大值为乙醇/乙醛的摩尔比为 1:1。本研究阐明了对现有机制的热力学见解,并推动了可行机制的演变。这项工作最终将有助于设计新型催化剂和优化工艺,使用来自可再生原料的乙醇进行可持续的生物基 BD 生产,这与全球对更绿色和资源友好型化学品制造的承诺保持一致。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号