Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-13 , DOI: 10.1002/adsc.202401295 Qiang Tang, Yanan Liu, Mengdi Wu, Yangzilin Kong, Xiaochun Jiang, Shizhang Ling, Yongjia Shang, Xinwei He
|
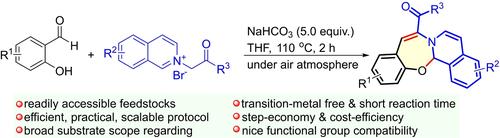
A concise and practical sustainable strategy for modular access to isoquinoline-fused benzo[f][1,3]oxazepine derivatives through a formal [4+3] annulation of commercially available salicyaldehydes with isoquinolinium salts has been developed. The reactions proceeded through the formation of two new bonds (C=C and C−O) and a seven-membered heterocyclic ring in one pot. Remarkably, use of simple NaHCO3 as a mild base, open atmosphere, nonhazardous reagents, nice functional group tolerance, and easily scale up are added characteristics to this approach. A wide range of substrates are compatible with this mild reaction system, thereby proving a facile and reliable protocol for constructing a benzo[f][1,3]oxazepine skeletons.
中文翻译:

NaHCO3 促进水杨醛与异喹啉盐的正式 [4+3] 环化获得异喹啉稠合苯并[f][1,3]氧氮卓类药物
已经开发了一种简洁实用的可持续策略,通过正式 [4+3] 市售水杨醛与异喹啉盐的环状化来模块化获取异喹啉熔融的苯并[f][1,3]恶氮西平衍生物。反应通过在一个罐中形成两个新键(C=C 和 C-O)和一个七元杂环进行。值得注意的是,使用简单的 NaHCO3 作为温和的碱、开放环境、无害试剂、良好的官能团耐受性和易于放大是该方法的附加特性。多种底物与这种温和的反应系统兼容,从而证明了构建苯并[f][1,3]恶氮平骨架的简单可靠的方案。































 京公网安备 11010802027423号
京公网安备 11010802027423号