当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium clusters decorated on lattice expanded hematite Fe2O3 for efficient electrocatalytic alkaline water splitting
Chemical Science ( IF 7.6 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4sc06732k Haibin Ma, Yongqiang Yang, Xiaohua Yu, Yang Zhao, Jiwei Ma, Hongfei Cheng
Chemical Science ( IF 7.6 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4sc06732k Haibin Ma, Yongqiang Yang, Xiaohua Yu, Yang Zhao, Jiwei Ma, Hongfei Cheng
|
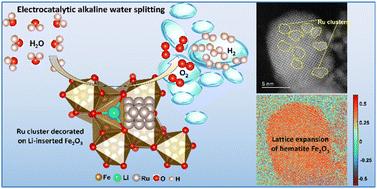
Electrocatalytic water splitting in alkaline media plays an important role in hydrogen production technology. Normally, the catalytic activity of commonly used transition metal oxides usually suffers from unsatisfactory electron conductivity and unfavorable binding strength for transition intermediates. To boost the intrinsic catalytic activity, we propose a rational strategy to construct lattice distorted transition metal oxides decorated with noble-metal nanoclusters. This strategy is verified by loading ruthenium clusters onto lithium ion intercalated hematite Fe2O3, which leads to significant distortion of the FeO6 unit cells. A remarkable overpotential of 21 mV with a Tafel slope of 39.8 mV dec−1 is achieved at 10 mA cm−2 for the hydrogen evolution reaction in 1.0 M KOH aqueous electrolyte. The assembled alkaline electrolyzer can catalyse overall water splitting for as long as 165 h at a current density of 250 mA cm−2 with negligible performance degradation, indicating great potential in the field of sustainable hydrogen production.
中文翻译:

钌团簇装饰在晶格膨胀赤铁矿 Fe2O3 上,用于高效电催化碱性水分解
碱性介质中的电催化分解水在制氢技术中起着重要作用。通常,常用过渡金属氧化物的催化活性通常受到电子电导率不令人满意和过渡中间体不利的结合强度的影响。为了提高本征催化活性,我们提出了一种合理的策略来构建用贵金属纳米团簇装饰的晶格扭曲过渡金属氧化物。通过将钌簇加载到锂离子插层赤铁矿 Fe2O3 上来验证这一策略,这会导致 FeO6 晶胞的显着变形。在 10 mA cm-2 处,对于 1.0 M KOH 水性电解质中的析氢反应,实现了 21 mV 的显着过电位和 39.8 mV dec-1 的塔菲尔斜率。组装好的碱性电解槽可以在 250 mA cm-2 的电流密度下催化整体分解水长达 165 小时,性能下降可以忽略不计,这表明在可持续氢气生产领域具有巨大潜力。
更新日期:2024-11-14
中文翻译:

钌团簇装饰在晶格膨胀赤铁矿 Fe2O3 上,用于高效电催化碱性水分解
碱性介质中的电催化分解水在制氢技术中起着重要作用。通常,常用过渡金属氧化物的催化活性通常受到电子电导率不令人满意和过渡中间体不利的结合强度的影响。为了提高本征催化活性,我们提出了一种合理的策略来构建用贵金属纳米团簇装饰的晶格扭曲过渡金属氧化物。通过将钌簇加载到锂离子插层赤铁矿 Fe2O3 上来验证这一策略,这会导致 FeO6 晶胞的显着变形。在 10 mA cm-2 处,对于 1.0 M KOH 水性电解质中的析氢反应,实现了 21 mV 的显着过电位和 39.8 mV dec-1 的塔菲尔斜率。组装好的碱性电解槽可以在 250 mA cm-2 的电流密度下催化整体分解水长达 165 小时,性能下降可以忽略不计,这表明在可持续氢气生产领域具有巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号