当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Synthesis of 2,2′-Biindoles via One-Pot Double Cycloisomerization Strategy
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.joc.4c01309 Jaime G. Ibarra-Gutiérrez, César R. Solorio-Alvarado, Luis Chacón-García, Jesús Adrián López, B. Yoaly Delgado-Piedra, Luis A. Segura-Quezada, Edson D. Hernández-Velázquez, Ana K. García-Dueñas
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.joc.4c01309 Jaime G. Ibarra-Gutiérrez, César R. Solorio-Alvarado, Luis Chacón-García, Jesús Adrián López, B. Yoaly Delgado-Piedra, Luis A. Segura-Quezada, Edson D. Hernández-Velázquez, Ana K. García-Dueñas
|
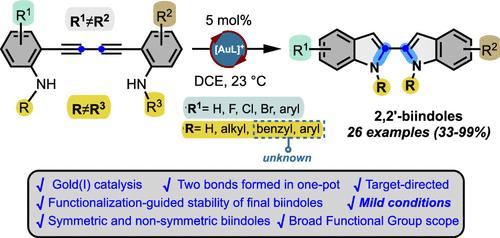
The first systematic, concise and target-directed gold(I)-catalyzed synthesis of a family of 2,2′-biindoles containing different substitution patterns is described. The developed protocol involves the synthesis of 1,3-diyne-anilines followed by a one-pot gold(I)-catalyzed double cycloisomerization, giving rise to an efficient, broad and general protocol to get different 2,2′-biindoles under mild reaction conditions. Due to the methodological restriction of present methods for accessing this class of compounds, herein we present our synthetic proposal which allowed the preparation of several examples of 2,2′-biindoles. Their functionalization-guided us to the discovery that the chemical stability, is substitution structure-dependent.
中文翻译:

金(I)催化通过一锅法双环异构化策略合成 2,2′-联吲哚
描述了包含不同取代模式的 2,2′-biindoles 家族的第一个系统、简洁和靶向导向的 gold(I) 催化合成。开发的方案涉及 1,3-二炔-苯胺的合成,然后进行一锅法金 (I) 催化的双环异构化,从而产生了一种高效、广泛和通用的方案,可在温和的反应条件下获得不同的 2,2′-联吲哚。由于目前访问此类化合物的方法学限制,我们在此提出我们的合成提案,该提案允许制备几个 2,2′-联吲哚的实例。他们的功能化引导我们发现化学稳定性是取代结构依赖性的。
更新日期:2024-11-14
中文翻译:

金(I)催化通过一锅法双环异构化策略合成 2,2′-联吲哚
描述了包含不同取代模式的 2,2′-biindoles 家族的第一个系统、简洁和靶向导向的 gold(I) 催化合成。开发的方案涉及 1,3-二炔-苯胺的合成,然后进行一锅法金 (I) 催化的双环异构化,从而产生了一种高效、广泛和通用的方案,可在温和的反应条件下获得不同的 2,2′-联吲哚。由于目前访问此类化合物的方法学限制,我们在此提出我们的合成提案,该提案允许制备几个 2,2′-联吲哚的实例。他们的功能化引导我们发现化学稳定性是取代结构依赖性的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号