当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Pyrrolidine-2-ylidenes from Isoxazolines and Allenes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.joc.4c01976 Dylan C. Keane, Guanqun Zhang, Abdullah S. Alshreimi, Donald J. Wink, Laura L. Anderson
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.joc.4c01976 Dylan C. Keane, Guanqun Zhang, Abdullah S. Alshreimi, Donald J. Wink, Laura L. Anderson
|
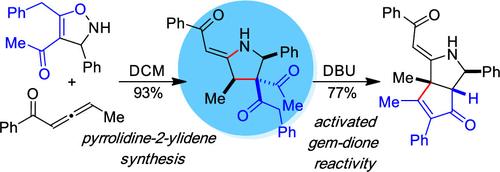
A diastereoselective addition and rearrangement reaction has been developed for the synthesis of pyrrolidine-2-ylidenes from NH-isoxazolines and electron-deficient allenes. This method proceeds via the rearrangement of a proposed N-alkenylisoxazoline intermediate to generate densely functionalized pyrrolidine-2-ylidenes under simple catalyst-free conditions that tolerate ketone substituents and install relative stereochemistry at positions 3 and 4 of the heterocycle. Reaction optimization and the substrate scope are described in addition to studies evaluating the reactivity of the gem-dione and enaminone groups of the products.
中文翻译:

由异恶唑啉和丙烯合成吡咯烷-2-亚基化物
已经开发了一种非对映选择性加成和重排反应,用于从 NH-异恶唑啉和缺电子丙烯合成吡咯烷-2-亚基化物。该方法通过重排拟议的 N-烯基异恶唑啉中间体进行,在简单的无催化剂条件下生成密集官能化的吡咯烷-2-亚酰亚胺,该条件耐受酮取代基并在杂环的位置 3 和 4 安装相对立体化学。除了评估产物的 gem-dione 和 enaminone 基团的反应性的研究外,还描述了反应优化和底物范围。
更新日期:2024-11-14
中文翻译:

由异恶唑啉和丙烯合成吡咯烷-2-亚基化物
已经开发了一种非对映选择性加成和重排反应,用于从 NH-异恶唑啉和缺电子丙烯合成吡咯烷-2-亚基化物。该方法通过重排拟议的 N-烯基异恶唑啉中间体进行,在简单的无催化剂条件下生成密集官能化的吡咯烷-2-亚酰亚胺,该条件耐受酮取代基并在杂环的位置 3 和 4 安装相对立体化学。除了评估产物的 gem-dione 和 enaminone 基团的反应性的研究外,还描述了反应优化和底物范围。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号