当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-d-Ribofuranose as a Core with a Phosphodiester Moiety as the Enzyme Recognition Site for Codrug Development
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03662 Jih Ru Hwu, Avijit Panja, Shwu-Chen Tsay, Wen-Chieh Huang, Shu-Yu Lin, Chen-Sheng Yeh, Wu-Chou Su, Li-Xing Yang, Dar-Bin Shieh
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03662 Jih Ru Hwu, Avijit Panja, Shwu-Chen Tsay, Wen-Chieh Huang, Shu-Yu Lin, Chen-Sheng Yeh, Wu-Chou Su, Li-Xing Yang, Dar-Bin Shieh
|
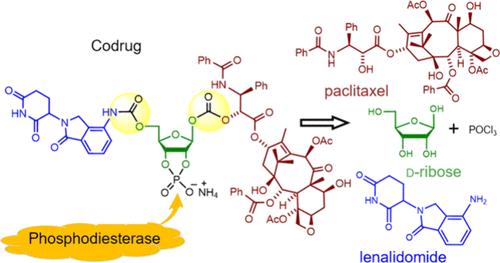
An ideal codrug design should be able to control drug release, offer selectivity during drug delivery, and break down into non-toxic fragments after biodegradation. Our design incorporates d-ribofuranose as the core, with carbamate and carbonate groups as linking joints, a phosphodiester moiety as an enzyme recognition site, and lenalidomide and paclitaxel as the constituent drugs. The codrug synthesis involves seven steps with a 33% overall yield. The target codrug increases its water solubility 685 times versus paclitaxel.
中文翻译:

β-d-核呋喃糖为核心,磷酸二酯部分为联合药物开发的酶识别位点
理想的联合药物设计应能够控制药物释放,在药物递送过程中提供选择性,并在生物降解后分解成无毒片段。我们的设计以 d-核糖为核心,以氨基甲酸酯和碳酸盐为连接关节,以磷酸二酯部分作为酶识别位点,来那度胺和紫杉醇为组成药物。联合药物合成涉及 7 个步骤,总产率为 33%。与紫杉醇相比,目标联合药物的水溶性增加了 685 倍。
更新日期:2024-11-14
中文翻译:

β-d-核呋喃糖为核心,磷酸二酯部分为联合药物开发的酶识别位点
理想的联合药物设计应能够控制药物释放,在药物递送过程中提供选择性,并在生物降解后分解成无毒片段。我们的设计以 d-核糖为核心,以氨基甲酸酯和碳酸盐为连接关节,以磷酸二酯部分作为酶识别位点,来那度胺和紫杉醇为组成药物。联合药物合成涉及 7 个步骤,总产率为 33%。与紫杉醇相比,目标联合药物的水溶性增加了 685 倍。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号