当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organic Photoredox Catalytic Difluoroalkylation of Unactivated Olefins to Access Difluoro-Containing Tetrahydropyridazines
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03829 Jun Sun, Yu Wei, Ting Lv, Chaodong Wang, Shengjie Song, Jiadi Zhou, Jianjun Li
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03829 Jun Sun, Yu Wei, Ting Lv, Chaodong Wang, Shengjie Song, Jiadi Zhou, Jianjun Li
|
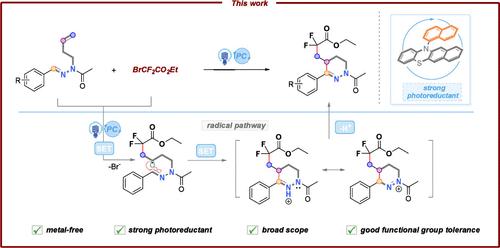
Herein, we disclose a readily available phenothiazine derivative as an organocatalyst, which upon excitation with 371 nm light acquires a strongly reducing power and serves to induce the radical cascade difluoromethylation/cyclization reaction of N-homoallylacetohydrazides. A variety of CF2COR-tetrahydropyridazines have been obtained in moderate to excellent yields. This catalytic platform proceeds under metal-free conditions with a wide substrate scope and broad functional group compatibility, which unlocks the new reactivity of phenothiazine derivatives and adds significant synthetic value to N-heterocycles.
中文翻译:

有机光氧化还原催化未活化烯烃的二氟烷基化反应得到含二氟的四氢吡嗪
在此,我们公开了一种现成的吩噻嗪衍生物作为有机催化剂,它在 371 nm 光激发下获得强还原能力,并用于诱导 N-均烯丙基酰肼的自由基级联二氟甲基化/环化反应。已获得多种 CF2COR-四氢吡嗪,收率中等至极高。该催化平台在无金属条件下进行,具有广泛的底物范围和广泛的官能团相容性,这释放了吩噻嗪衍生物的新反应性,并为 N-杂环增加了显着的合成价值。
更新日期:2024-11-14
中文翻译:

有机光氧化还原催化未活化烯烃的二氟烷基化反应得到含二氟的四氢吡嗪
在此,我们公开了一种现成的吩噻嗪衍生物作为有机催化剂,它在 371 nm 光激发下获得强还原能力,并用于诱导 N-均烯丙基酰肼的自由基级联二氟甲基化/环化反应。已获得多种 CF2COR-四氢吡嗪,收率中等至极高。该催化平台在无金属条件下进行,具有广泛的底物范围和广泛的官能团相容性,这释放了吩噻嗪衍生物的新反应性,并为 N-杂环增加了显着的合成价值。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号