当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed Regioselective C–H Alkenylation and Alkylation of Menadione Analogues with β-Trifluoromethyl Enones
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03857 Haritha Sindhe, Anand Kumar, Haneesha Gulipelli, Akshay Kamble, Amardeep Singh, Satyasheel Sharma
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-14 , DOI: 10.1021/acs.orglett.4c03857 Haritha Sindhe, Anand Kumar, Haneesha Gulipelli, Akshay Kamble, Amardeep Singh, Satyasheel Sharma
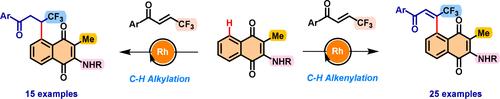
|
Menadione and its structural analogues are important motifs present in various bioactive natural products and drugs with a wide range of biological activities. In addition, β-trifluoromethyl enone has been employed as an efficient fluorinated building block for the synthesis of CF3-containing organic scaffolds. Herein, we report both C–H alkenylation and C–H alkylation reactions using β-CF3 enones as coupling partners with amino-substituted menadiones under Rh(III) catalysis. While β-CF3 enones have been studied in C–H alkylation reactions, we herein for the first time disclose the Rh (III)-catalyzed C–H alkenylation reaction of β-CF3 enones. The presence of an oxidant was crucial for the alkenylation reaction with β-CF3 enone. Meanwhile, the alkylation reaction proceeds via redox-neutral conditions.
中文翻译:

铑 (III) 催化的甲萘醌类似物与 β-三氟甲基烯酮的区域选择性 C-H 烯基化和烷基化
甲萘醌及其结构类似物是存在于各种生物活性天然产物和具有广泛生物活性的药物中的重要基序。此外,β-三氟甲基烯酮已被用作合成含 CF3 有机支架的有效氟化结构单元。在此,我们报道了在 Rh(III) 催化下使用 β-CF3 烯酮作为偶联伴侣与氨基取代的甲萘酮的 C-H 烯基化和 C-H 烷基化反应。虽然已经在 C-H 烷基化反应中研究了 β-CF3 烯酮,但我们在此首次披露了 Rh (III) 催化的 β-CF3 烯基化反应。氧化剂的存在对于与 β-CF3 烯酮的烯基化反应至关重要。同时,烷基化反应通过氧化还原中性条件进行。
更新日期:2024-11-14
中文翻译:

铑 (III) 催化的甲萘醌类似物与 β-三氟甲基烯酮的区域选择性 C-H 烯基化和烷基化
甲萘醌及其结构类似物是存在于各种生物活性天然产物和具有广泛生物活性的药物中的重要基序。此外,β-三氟甲基烯酮已被用作合成含 CF3 有机支架的有效氟化结构单元。在此,我们报道了在 Rh(III) 催化下使用 β-CF3 烯酮作为偶联伴侣与氨基取代的甲萘酮的 C-H 烯基化和 C-H 烷基化反应。虽然已经在 C-H 烷基化反应中研究了 β-CF3 烯酮,但我们在此首次披露了 Rh (III) 催化的 β-CF3 烯基化反应。氧化剂的存在对于与 β-CF3 烯酮的烯基化反应至关重要。同时,烷基化反应通过氧化还原中性条件进行。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号