Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
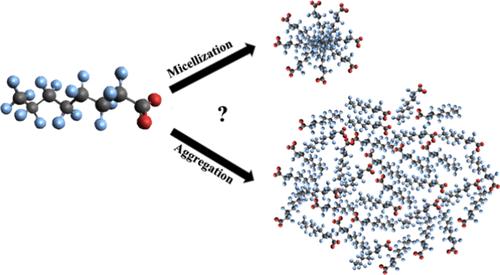
Aggregation, Not Micellization: Perfluorooctanoic Acid, Perfluorobutanesulfonic Acid, and Potassium Perfluorooctanesulfonate Behavior in Aqueous Solution
Langmuir ( IF 3.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.langmuir.4c02566 Tess N. Sobolewski, J. Luke Findlay, Jackilyn E. Hemphill, Robert A. Walker
Langmuir ( IF 3.7 ) Pub Date : 2024-11-13 , DOI: 10.1021/acs.langmuir.4c02566 Tess N. Sobolewski, J. Luke Findlay, Jackilyn E. Hemphill, Robert A. Walker
|
Surface tension, conductivity, and dynamic light scattering (DLS) measurements were used to examine the surface and bulk solution behaviors of three members of the PFAS family, perfluorooctanoic acid (PFOA), perfluorobutanesulfonic acid (PFBS), and the potassium salt of perfluorooctanesulfonic acid (PFOS). Measurements were carried out in solutions having variable (acidic) pH and in solutions buffered to pH = 8.0. Surface tension data show traditional soluble surfactant behavior, and results illustrate that PFOA, PFBS, and PFOS surface activity depends sensitively on solution phase pH. The tightly packed monolayers formed by PFOA in mildly acidic solutions imply that the surface pH of PFOA solutions is several units lower than bulk. Results from conductivity experiments generally show increasing conductivity with increasing bulk solution surfactant concentration. In pH = 8.0 solutions, changes in conductivity slope with surfactant concentration suggest the onset of micelle formation at concentrations <1 mM, markedly lower than reported in literature. In general, apparent critical micelle concentrations (CMCs) determined from conductivity data agree with similar predictions made from surface tension results. DLS measurements show that at concentrations close to the predicted PFAS CMCs, objects with diameters ≤10 nm start to form. However, unlike micelles, these objects continue to grow with increasing bulk solute concentration. These aggregates form structures having diameters of 50–150 nm. Aggregate size shrinks modestly as solution phase temperature increases, and this behavior is reversible. Cryo-EM images of PFOA solutions confirm a broad distribution of particles, supporting the DLS measurements. Findings reported in this work represent the first evidence that these three EPA-regulated PFAS surfactants form aggregates rather than micelles in solution. Findings also begin to reconcile differences in reported surface behaviors that have led to CMC predictions in the literature varying by more than an order of magnitude.
中文翻译:

聚集,而不是胶束化:全氟辛酸、全氟丁烷磺酸和全氟辛烷磺酸钾在水溶液中的行为
表面张力、电导率和动态光散射 (DLS) 测量用于检查 PFAS 家族三个成员的表面和本体溶液行为,即全氟辛酸 (PFOA)、全氟丁烷磺酸 (PFBS) 和全氟辛烷磺酸 (PFOS) 的钾盐。在具有可变(酸性)pH 值的溶液和缓冲至 pH = 8.0 的溶液中进行测量。表面张力数据显示了传统的可溶性表面活性剂行为,结果表明 PFOA、PFBS 和 PFOS 表面活性对液相 pH 值敏感。PFOA 在弱酸性溶液中形成的紧密堆积单层意味着 PFOA 溶液的表面 pH 值比本体低几个单位。电导率实验的结果通常显示,电导率随着本体溶液表面活性剂浓度的增加而增加。在 pH = 8.0 溶液中,电导率斜率随表面活性剂浓度的变化表明胶束形成的浓度为 <1 mM,明显低于文献中报道的浓度。一般来说,根据电导率数据确定的表观临界胶束浓度 (CMC) 与根据表面张力结果做出的类似预测一致。DLS 测量表明,当浓度接近预测的 PFAS CMC 时,直径为 ≤10 nm 的物体开始形成。然而,与胶束不同的是,这些物体随着体积溶质浓度的增加而继续生长。这些聚集体形成直径为 50-150 nm 的结构。随着固相温度的升高,聚集体尺寸会适度缩小,并且这种行为是可逆的。PFOA 溶液的冷冻电镜图像证实了颗粒的广泛分布,支持 DLS 测量。 这项工作中报告的研究结果代表了这三种 EPA 监管的 PFAS 表面活性剂在溶液中形成聚集体而不是胶束的第一个证据。研究结果还开始调和报告的表面行为的差异,这些差异导致文献中 CMC 预测的变化超过一个数量级。
更新日期:2024-11-14
中文翻译:

聚集,而不是胶束化:全氟辛酸、全氟丁烷磺酸和全氟辛烷磺酸钾在水溶液中的行为
表面张力、电导率和动态光散射 (DLS) 测量用于检查 PFAS 家族三个成员的表面和本体溶液行为,即全氟辛酸 (PFOA)、全氟丁烷磺酸 (PFBS) 和全氟辛烷磺酸 (PFOS) 的钾盐。在具有可变(酸性)pH 值的溶液和缓冲至 pH = 8.0 的溶液中进行测量。表面张力数据显示了传统的可溶性表面活性剂行为,结果表明 PFOA、PFBS 和 PFOS 表面活性对液相 pH 值敏感。PFOA 在弱酸性溶液中形成的紧密堆积单层意味着 PFOA 溶液的表面 pH 值比本体低几个单位。电导率实验的结果通常显示,电导率随着本体溶液表面活性剂浓度的增加而增加。在 pH = 8.0 溶液中,电导率斜率随表面活性剂浓度的变化表明胶束形成的浓度为 <1 mM,明显低于文献中报道的浓度。一般来说,根据电导率数据确定的表观临界胶束浓度 (CMC) 与根据表面张力结果做出的类似预测一致。DLS 测量表明,当浓度接近预测的 PFAS CMC 时,直径为 ≤10 nm 的物体开始形成。然而,与胶束不同的是,这些物体随着体积溶质浓度的增加而继续生长。这些聚集体形成直径为 50-150 nm 的结构。随着固相温度的升高,聚集体尺寸会适度缩小,并且这种行为是可逆的。PFOA 溶液的冷冻电镜图像证实了颗粒的广泛分布,支持 DLS 测量。 这项工作中报告的研究结果代表了这三种 EPA 监管的 PFAS 表面活性剂在溶液中形成聚集体而不是胶束的第一个证据。研究结果还开始调和报告的表面行为的差异,这些差异导致文献中 CMC 预测的变化超过一个数量级。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号