当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Early-stage oxidation and subsequent damage of the used nuclear fuel extractant TODGA; electron pulse radiolysis and theoretical insights
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4cp03678f Rupali G. Deokar, Andrew R. Cook
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-14 , DOI: 10.1039/d4cp03678f Rupali G. Deokar, Andrew R. Cook
|
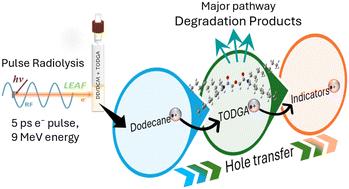
Radiation induced damage of extractant molecules is a well-known phenomenon responsible for reducing efficiency and increasing the waste and cost of reprocessing used nuclear fuel (UNF). As such, understanding early-stage (pico- to nanoseconds) radiation-induced reaction mechanisms is essential for informing the design of next generation extractants with enhanced radiation robustness. Here we utilized picosecond and nanosecond electron pulse radiolysis experiments to probe the early-stage radioactive environment experienced by the organic phase extractant N,N,N′,N′-tetraoctyldiglycolamide (TODGA), proposed for separating highly radioactive trivalent minor actinides (specifically americium and curium) from the trivalent lanthanides. Using comparisons to the similar ionization potential (IP) solute p-xylene, this work determined the mechanism of reaction with the ionized diluent (i.e., n-dodecane radical cation, DD˙+) is hole transfer to produce TODGA˙+. At high TODGA concentrations (>100 mM), the majority of this transfer occurs faster than 10 ps via the capture of DD˙+ holes prior to their solvation with a C37 = 300 mM. The surviving solvated holes were captured with k = (2.38 ± 0.15) × 1010 M−1 s−1. Attempts at subsequent hole transfer to lower IP solutes found that only 10% of holes were transferred, indicating bond rupture of TODGA˙+ occurs within 2.6 ns at 200 mM TODGA. Possible reaction pathways for the rapid decomposition of TODGA˙+ were explored using a combination of experiments and density functional theory (DFT) calculations.
中文翻译:

乏核燃料萃取剂 TODGA 的早期氧化和后续损坏;电子脉冲放射分解和理论见解
辐射引起的萃取剂分子损伤是一种众所周知的现象,会导致效率降低并增加乏核燃料 (UNF) 后处理的废物和成本。因此,了解早期(皮秒到纳秒)辐射诱导的反应机制对于设计具有增强辐射稳健性的下一代萃取剂至关重要。在这里,我们利用皮秒和纳秒电子脉冲辐射分解实验来探测有机相萃取剂 N,N,N′,N′-四辛基二甘酰胺 (TODGA) 所经历的早期放射性环境,该萃取剂被提议用于从三价镧系元素中分离高放射性三价次锕系元素(特别是镅和锔)。通过与类似的电离电位 (IP) 溶质对二甲苯进行比较,本研究确定了与电离稀释剂(即正十二烷自由基阳离子,DD ̇+)的反应机理是空穴转移产生 TODGA ̇+。在高 TODGA 浓度 (>100 mM) 下,通过在 C37 = 300 mM 溶剂化之前捕获 DD ̇+ 空穴,大部分转移发生得比 10 ps 快。用 k = (2.38 ± 0.15) × 1010 M-1 s-1 捕获幸存的溶剂化空穴。随后尝试将空穴转移到较低 IP 溶质中,发现只有 10% 的空穴被转移,这表明 TODGA ̇+ 的键断裂发生在 2.6 mM TODGA 的 2.6 ns 内。 通过结合实验和密度泛函理论 (DFT) 计算,探索了 TODGA ̇+ 快速分解的可能反应途径。
更新日期:2024-11-18
中文翻译:

乏核燃料萃取剂 TODGA 的早期氧化和后续损坏;电子脉冲放射分解和理论见解
辐射引起的萃取剂分子损伤是一种众所周知的现象,会导致效率降低并增加乏核燃料 (UNF) 后处理的废物和成本。因此,了解早期(皮秒到纳秒)辐射诱导的反应机制对于设计具有增强辐射稳健性的下一代萃取剂至关重要。在这里,我们利用皮秒和纳秒电子脉冲辐射分解实验来探测有机相萃取剂 N,N,N′,N′-四辛基二甘酰胺 (TODGA) 所经历的早期放射性环境,该萃取剂被提议用于从三价镧系元素中分离高放射性三价次锕系元素(特别是镅和锔)。通过与类似的电离电位 (IP) 溶质对二甲苯进行比较,本研究确定了与电离稀释剂(即正十二烷自由基阳离子,DD ̇+)的反应机理是空穴转移产生 TODGA ̇+。在高 TODGA 浓度 (>100 mM) 下,通过在 C37 = 300 mM 溶剂化之前捕获 DD ̇+ 空穴,大部分转移发生得比 10 ps 快。用 k = (2.38 ± 0.15) × 1010 M-1 s-1 捕获幸存的溶剂化空穴。随后尝试将空穴转移到较低 IP 溶质中,发现只有 10% 的空穴被转移,这表明 TODGA ̇+ 的键断裂发生在 2.6 mM TODGA 的 2.6 ns 内。 通过结合实验和密度泛函理论 (DFT) 计算,探索了 TODGA ̇+ 快速分解的可能反应途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号